Advances in NK cell production
- PMID: 34983953
- PMCID: PMC8975878
- DOI: 10.1038/s41423-021-00808-3
Advances in NK cell production
Abstract
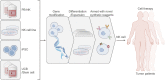
Immunotherapy based on natural killer (NK) cells is a promising approach for treating a variety of cancers. Unlike T cells, NK cells recognize target cells via a major histocompatibility complex (MHC)-independent mechanism and, without being sensitized, kill the cells directly. Several strategies for obtaining large quantities of NK cells with high purity and high cytotoxicity have been developed. These strategies include the use of cytokine-antibody fusions, feeder cells or membrane particles to stimulate the proliferation of NK cells and enhance their cytotoxicity. Various materials, including peripheral blood mononuclear cells (PBMCs), umbilical cord blood (UCB), induced pluripotent stem cells (iPSCs) and NK cell lines, have been used as sources to generate NK cells for immunotherapy. Moreover, genetic modification technologies to improve the proliferation of NK cells have also been developed to enhance the functions of NK cells. Here, we summarize the recent advances in expansion strategies with or without genetic manipulation of NK cells derived from various cellular sources. We also discuss the closed, automated and GMP-controlled large-scale expansion systems used for NK cells and possible future NK cell-based immunotherapy products.
Keywords: Genetic manipulation; NK cell lines; NK cells; PBMCs; UCB; iPSCs.
© 2021. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures
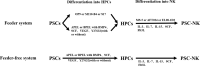


Similar articles
-
A novel method to expand large numbers of CD56(+) natural killer cells from a minute fraction of selectively accessed cryopreserved cord blood for immunotherapy after transplantation.Cytotherapy. 2015 Nov;17(11):1582-93. doi: 10.1016/j.jcyt.2015.07.020. Cytotherapy. 2015. PMID: 26432560 Free PMC article.
-
Feeder-Cell-Free and Serum-Free Expansion of Natural Killer Cells Using Cloudz Microspheres, G-Rex6M, and Human Platelet Lysate.Front Immunol. 2022 Mar 7;13:803380. doi: 10.3389/fimmu.2022.803380. eCollection 2022. Front Immunol. 2022. PMID: 35320938 Free PMC article.
-
High log-scale expansion of functional human natural killer cells from umbilical cord blood CD34-positive cells for adoptive cancer immunotherapy.PLoS One. 2010 Feb 15;5(2):e9221. doi: 10.1371/journal.pone.0009221. PLoS One. 2010. PMID: 20169160 Free PMC article.
-
Cord-Blood Natural Killer Cell-Based Immunotherapy for Cancer.Front Immunol. 2020 Oct 22;11:584099. doi: 10.3389/fimmu.2020.584099. eCollection 2020. Front Immunol. 2020. PMID: 33193399 Free PMC article. Review.
-
Selection and expansion of natural killer cells for NK cell-based immunotherapy.Cancer Immunol Immunother. 2016 Apr;65(4):477-84. doi: 10.1007/s00262-016-1792-y. Epub 2016 Jan 25. Cancer Immunol Immunother. 2016. PMID: 26810567 Free PMC article. Review.
Cited by
-
Methodological Approaches for Increasing the Retroviral Transduction Efficiency of Primary NK Cells.Curr Pharm Des. 2024;30(37):2947-2958. doi: 10.2174/0113816128314633240724060916. Curr Pharm Des. 2024. PMID: 39136515
-
CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances.Mol Cancer. 2023 Jan 30;22(1):20. doi: 10.1186/s12943-023-01723-z. Mol Cancer. 2023. PMID: 36717905 Free PMC article. Review.
-
Natural killer cell-based cancer immunotherapy: from basics to clinical trials.Exp Hematol Oncol. 2024 Oct 16;13(1):101. doi: 10.1186/s40164-024-00561-z. Exp Hematol Oncol. 2024. PMID: 39415291 Free PMC article. Review.
-
Beyond CAR-T: The rise of CAR-NK cell therapy in asthma immunotherapy.J Transl Med. 2024 Aug 5;22(1):736. doi: 10.1186/s12967-024-05534-8. J Transl Med. 2024. PMID: 39103889 Free PMC article. Review.
-
Panoramic comparison between NK cells in healthy and cancerous liver through single-cell RNA sequencing.Cancer Biol Med. 2022 Jul 21;19(9):1334-51. doi: 10.20892/j.issn.2095-3941.2022.0050. Cancer Biol Med. 2022. PMID: 35856557 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

