Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: a retrospective analysis of active surveillance database
- PMID: 34983406
- PMCID: PMC8724590
- DOI: 10.1186/s12879-021-07004-8
Safety and clinical outcomes of remdesivir in hospitalised COVID-19 patients: a retrospective analysis of active surveillance database
Abstract
Background: Real-world data on safety and clinical outcomes of remdesivir in COVID-19 management is scant. We present findings of data analysis conducted for assessing the safety and clinical outcomes of remdesivir treatment for COVID-19 in India.
Methods: This retrospective analysis used data from an active surveillance programme database of hospitalised patients with COVID-19 who were receiving remdesivir.
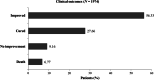
Results: Of the 2329 patients included, 67.40% were men. Diabetes (29.69%) and hypertension (20.33%) were the most common comorbidities. At remdesivir initiation, 2272 (97.55%) patients were receiving oxygen therapy. Remdesivir was administered for 5 days in 65.38% of patients. Antibiotics (64.90%) and steroids (47.90%) were the most common concomitant medications. Remdesivir was overall well tolerated, and total 119 adverse events were reported; most common were nausea and vomiting in 45.40% and increased liver enzymes in 14.28% patients. 84% of patients were cured/improved, 6.77% died and 9.16% showed no improvement in their clinical status at data collection. Subgroup analyses showed that the mortality rate was significantly lower in patients < 60 years old than in those > 60 years old. Amongst patients on oxygen therapy, the cure/improvement rate was significantly higher in those receiving standard low-flow oxygen than in those receiving mechanical ventilation, non-invasive ventilation, or high-flow oxygen. Factors that were associated with higher mortality were age > 60 years, cardiac disease, diabetes high flow oxygen, non-invasive ventilation and mechanical ventilation.
Conclusion: Our analysis showed that remdesivir is well tolerated and has an acceptable safety profile. The clinical outcome of cure/improvement was 84%, with a higher improvement in patients < 60 years old and on standard low-flow oxygen.
Keywords: Active surveillance; COVID-19; Remdesivir; Retrospective analysis; Retrospective studies.
© 2021. The Author(s).
Conflict of interest statement
All the authors are permanent employees of Cipla Ltd., India who had a role in the study. The study was funded by Cipla Ltd., India, and was the provider of the drug.
Similar articles
-
Remdesivir for the Treatment of Severe COVID-19: A Community Hospital's Experience.J Am Osteopath Assoc. 2020 Dec 1;120(12):926-933. doi: 10.7556/jaoa.2020.156. J Am Osteopath Assoc. 2020. PMID: 33136164
-
The effect of early remdesivir administration in COVID-19 disease progression in hospitalised patients.Wien Klin Wochenschr. 2024 Aug;136(15-16):458-464. doi: 10.1007/s00508-024-02377-7. Epub 2024 Jun 17. Wien Klin Wochenschr. 2024. PMID: 38884783 Free PMC article.
-
Experience in the use of remdesivir in patients with SARS-CoV-2 pneumonia.Farm Hosp. 2021 Aug 2;45(5):253-257. Farm Hosp. 2021. PMID: 34806585 English.
-
Efficacy and safety of remdesivir in hospitalised COVID-19 patients: a systematic review and meta-analysis.Infection. 2022 Feb;50(1):27-41. doi: 10.1007/s15010-021-01671-0. Epub 2021 Jul 31. Infection. 2022. PMID: 34331674 Free PMC article. Review.
-
Remdesivir for the treatment of COVID 19: review of the pharmacological properties, safety and clinical effectiveness.Expert Opin Drug Saf. 2021 Nov;20(11):1299-1307. doi: 10.1080/14740338.2021.1962284. Epub 2021 Aug 16. Expert Opin Drug Saf. 2021. PMID: 34338121 Review.
Cited by
-
Remdesivir-induced bradycardia in a 26-year-old patient with COVID-19: a case report.Infection. 2022 Dec;50(6):1605-1613. doi: 10.1007/s15010-022-01854-3. Epub 2022 Jun 14. Infection. 2022. PMID: 35701724 Free PMC article.
-
The Efficacy and Safety of Imusil® Tablets in the Treatment of Adult Patients With Mild COVID-19: A Prospective, Randomized, Multicenter, Open-Label Study.Cureus. 2023 Mar 7;15(3):e35881. doi: 10.7759/cureus.35881. eCollection 2023 Mar. Cureus. 2023. PMID: 37051002 Free PMC article.
-
Immune-related therapeutics: an update on antiviral drugs and vaccines to tackle the COVID-19 pandemic.Osong Public Health Res Perspect. 2022 Apr;13(2):84-100. doi: 10.24171/j.phrp.2022.0024. Epub 2022 Apr 27. Osong Public Health Res Perspect. 2022. PMID: 35538681 Free PMC article.
-
Efficacy of Kan Jang® in Patients with Mild COVID-19: A Randomized, Quadruple-Blind, Placebo-Controlled Trial.Pharmaceuticals (Basel). 2023 Aug 22;16(9):1196. doi: 10.3390/ph16091196. Pharmaceuticals (Basel). 2023. PMID: 37765004 Free PMC article.
-
A Prospective Study of AFree Oral Spray as an Adjuvant Therapy for Mild and Moderate COVID-19 in Community Health Stations: Clinical Progression and Viral Clearance Outcomes.In Vivo. 2023 Sep-Oct;37(5):2155-2160. doi: 10.21873/invivo.13313. In Vivo. 2023. PMID: 37652493 Free PMC article. Clinical Trial.
References
-
- WHO Coronavirus (COVID-19) Dashboard. URL: https://covid19.who.int/. Accessed 02 June 2021.
-
- Kupferschmidt K, Cohen J. WHO launches global megatrial of the four most promising coronavirus treatments. Science. 2020. https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-fo.... Accessed 17 March 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources