Neuraminidase1 Inhibitor Protects Against Doxorubicin-Induced Cardiotoxicity via Suppressing Drp1-Dependent Mitophagy
- PMID: 34977042
- PMCID: PMC8719652
- DOI: 10.3389/fcell.2021.802502
Neuraminidase1 Inhibitor Protects Against Doxorubicin-Induced Cardiotoxicity via Suppressing Drp1-Dependent Mitophagy
Abstract
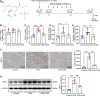
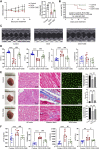
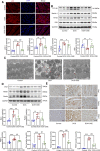
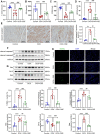
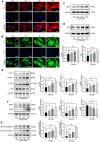
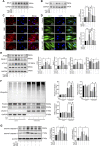
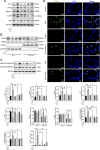
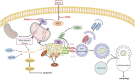
Anthracyclines, such as doxorubicin (DOX), are among the effective chemotherapeutic drugs for various malignancies. However, their clinical use is limited by irreversible cardiotoxicity. This study sought to determine the role of neuraminidase 1 (NEU1) in DOX-induced cardiomyopathy and the potential cardio-protective effects of NEU1 inhibitor oseltamivir (OSE). Male Sprague-Dawley (SD) rats were randomized into three groups: control, DOX, and DOX + OSE. NEU1 was highly expressed in DOX-treated rat heart tissues compared with the control group, which was suppressed by OSE administration. Rats in the DOX + OSE group showed preserved cardiac function and were protected from DOX-induced cardiomyopathy. The beneficial effects of OSE were associated with the suppression of dynamin-related protein 1 (Drp1)-dependent mitochondrial fission and mitophagy. In detail, the elevated NEU1 in cardiomyocytes triggered by DOX increased the expression of Drp1, which subsequently enhanced mitochondrial fission and PINK1/Parkin pathway-mediated mitophagy, leading to a maladaptive feedback circle towards myocardial apoptosis and cell death. OSE administration selectively inhibited the increased NEU1 in myocardial cells insulted by DOX, followed by reduction of Drp1 expression, inhibition of PINK1 stabilization on mitochondria, and Parkin translocation to mitochondria, thus alleviating excessive mitochondrial fission and mitophagy, alleviating subsequent development of cellular apoptotic process. This work identified NEU1 as a crucial inducer of DOX-induced cardiomyopathy by promoting Drp1-dependent mitochondrial fission and mitophagy, and NEU1 inhibitor showed new indications of cardio-protection against DOX cardiotoxicity.
Keywords: cardiotoxicity; doxorubicin; dynamin-related protein 1 (Drp1); mitophagy; neuraminidase1; oseltamivir.
Copyright © 2021 Qin, Lv, Zhang, Ruan, Xu, Chen, Ji, Lu and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of Drp1-mediated maladaptive mitochondrial fission.Pharmacol Res. 2020 Jul;157:104846. doi: 10.1016/j.phrs.2020.104846. Epub 2020 Apr 25. Pharmacol Res. 2020. PMID: 32339784
-
Doxorubicin-induced cardiomyocyte death is mediated by unchecked mitochondrial fission and mitophagy.FASEB J. 2019 Oct;33(10):11096-11108. doi: 10.1096/fj.201802663R. Epub 2019 Jul 10. FASEB J. 2019. PMID: 31291545 Free PMC article.
-
SESN2 protects against doxorubicin-induced cardiomyopathy via rescuing mitophagy and improving mitochondrial function.J Mol Cell Cardiol. 2019 Aug;133:125-137. doi: 10.1016/j.yjmcc.2019.06.005. Epub 2019 Jun 12. J Mol Cell Cardiol. 2019. PMID: 31199952
-
Mitochondrial Dynamin-Related Protein Drp1: a New Player in Cardio-oncology.Curr Oncol Rep. 2022 Dec;24(12):1751-1763. doi: 10.1007/s11912-022-01333-w. Epub 2022 Oct 1. Curr Oncol Rep. 2022. PMID: 36181612 Free PMC article. Review.
-
Targeting mitochondrial dynamics proteins for the treatment of doxorubicin-induced cardiotoxicity.Front Mol Biosci. 2023 Aug 3;10:1241225. doi: 10.3389/fmolb.2023.1241225. eCollection 2023. Front Mol Biosci. 2023. PMID: 37602332 Free PMC article. Review.
Cited by
-
The Role of Mitochondrial Quality Control in Anthracycline-Induced Cardiotoxicity: From Bench to Bedside.Oxid Med Cell Longev. 2022 Sep 21;2022:3659278. doi: 10.1155/2022/3659278. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36187332 Free PMC article. Review.
-
Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms, Clinical Management and Innovative Treatment.Drug Des Devel Ther. 2024 Sep 12;18:4089-4116. doi: 10.2147/DDDT.S469331. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39286288 Free PMC article. Review.
-
Recent Advances in Mitochondrial Fission/Fusion-Targeted Therapy in Doxorubicin-Induced Cardiotoxicity.Pharmaceutics. 2023 Apr 7;15(4):1182. doi: 10.3390/pharmaceutics15041182. Pharmaceutics. 2023. PMID: 37111670 Free PMC article. Review.
-
Role of mitochondria in doxorubicin-mediated cardiotoxicity: from molecular mechanisms to therapeutic strategies.Int J Med Sci. 2024 Mar 11;21(5):809-816. doi: 10.7150/ijms.94485. eCollection 2024. Int J Med Sci. 2024. PMID: 38617011 Free PMC article. Review.
-
Mitochondria and Doxorubicin-Induced Cardiomyopathy: A Complex Interplay.Cells. 2022 Jun 22;11(13):2000. doi: 10.3390/cells11132000. Cells. 2022. PMID: 35805084 Free PMC article. Review.
References
-
- Ambrosone C. B., Zirpoli G. R., Hutson A. D., McCann W. E., McCann S. E., Barlow W. E., et al. (2020). Dietary Supplement Use during Chemotherapy and Survival Outcomes of Patients with Breast Cancer Enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J. Clin. Oncol. 38 (8), 804–814. 10.1200/jco.19.01203 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

