Development of a Novel Perfusable Solution for ex vivo Preservation: Towards Photosynthetic Oxygenation for Organ Transplantation
- PMID: 34976984
- PMCID: PMC8714958
- DOI: 10.3389/fbioe.2021.796157
Development of a Novel Perfusable Solution for ex vivo Preservation: Towards Photosynthetic Oxygenation for Organ Transplantation
Abstract
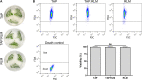
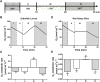
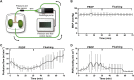
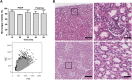
Oxygen is the key molecule for aerobic metabolism, but no animal cells can produce it, creating an extreme dependency on external supply. In contrast, microalgae are photosynthetic microorganisms, therefore, they are able to produce oxygen as plant cells do. As hypoxia is one of the main issues in organ transplantation, especially during preservation, the main goal of this work was to develop the first generation of perfusable photosynthetic solutions, exploring its feasibility for ex vivo organ preservation. Here, the microalgae Chlamydomonas reinhardtii was incorporated in a standard preservation solution, and key aspects such as alterations in cell size, oxygen production and survival were studied. Osmolarity and rheological features of the photosynthetic solution were comparable to human blood. In terms of functionality, the photosynthetic solution proved to be not harmful and to provide sufficient oxygen to support the metabolic requirement of zebrafish larvae and rat kidney slices. Thereafter, isolated porcine kidneys were perfused, and microalgae reached all renal vasculature, without inducing damage. After perfusion and flushing, no signs of tissue damage were detected, and recovered microalgae survived the process. Altogether, this work proposes the use of photosynthetic microorganisms as vascular oxygen factories to generate and deliver oxygen in isolated organs, representing a novel and promising strategy for organ preservation.
Keywords: Chlamydomonas reinhardtii; hypoxia; ischemia; organ perfusion; organ preservation; photosynthesis; photosynthetic microorganisms.
Copyright © 2021 Veloso-Giménez, Escamilla, Necuñir, Corrales-Orovio, Riveros, Marino, Ehrenfeld, Guzmán, Boric, Rebolledo and Egaña.
Conflict of interest statement
Author CG was employed by company Sky-Walker SpA. Competing Interests: JTE is CSO and co-founder of SymbiOx Inc., a start-up company that owns IP in the field of this work. Thanks to an R and D grant provided by the Chilean Ministry of Economics (CORFO), during the conduct of this project, DN, RE and RC-O were full-time employees of SymbiOx Inc. All other authors declare that they have no competing interests.
Figures



Similar articles
-
Towards an In Vitro 3D Model for Photosynthetic Cancer Treatment: A Study of Microalgae and Tumor Cell Interactions.Int J Mol Sci. 2022 Nov 4;23(21):13550. doi: 10.3390/ijms232113550. Int J Mol Sci. 2022. PMID: 36362338 Free PMC article.
-
Towards chlorocytes for therapeutic intravascular photosynthesis.Appl Microbiol Biotechnol. 2024 Oct 17;108(1):489. doi: 10.1007/s00253-024-13285-1. Appl Microbiol Biotechnol. 2024. PMID: 39417888 Free PMC article. Review.
-
Microalgae share key features with human erythrocytes and can safely circulate through the vascular system in mice.Appl Microbiol Biotechnol. 2023 Jul;107(14):4621-4633. doi: 10.1007/s00253-023-12588-z. Epub 2023 May 25. Appl Microbiol Biotechnol. 2023. PMID: 37227473
-
Photosynthetic biomaterials: a pathway towards autotrophic tissue engineering.Acta Biomater. 2015 Mar;15:39-47. doi: 10.1016/j.actbio.2014.12.012. Epub 2014 Dec 20. Acta Biomater. 2015. PMID: 25536030
-
Ex Vivo Porcine Organ Perfusion Models as a Suitable Platform for Translational Transplant Research.Artif Organs. 2017 Sep;41(9):E69-E79. doi: 10.1111/aor.12865. Epub 2017 Mar 6. Artif Organs. 2017. PMID: 28266040 Review.
Cited by
-
Towards an In Vitro 3D Model for Photosynthetic Cancer Treatment: A Study of Microalgae and Tumor Cell Interactions.Int J Mol Sci. 2022 Nov 4;23(21):13550. doi: 10.3390/ijms232113550. Int J Mol Sci. 2022. PMID: 36362338 Free PMC article.
-
Towards chlorocytes for therapeutic intravascular photosynthesis.Appl Microbiol Biotechnol. 2024 Oct 17;108(1):489. doi: 10.1007/s00253-024-13285-1. Appl Microbiol Biotechnol. 2024. PMID: 39417888 Free PMC article. Review.
-
Case report: Long-term follow-up of a large full-thickness skin defect treated with a photosynthetic scaffold for dermal regeneration.Front Bioeng Biotechnol. 2022 Dec 1;10:1004155. doi: 10.3389/fbioe.2022.1004155. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36532582 Free PMC article.
-
Dual roles of photosynthetic hydrogel with sustained oxygen generation in promoting cell survival and eradicating anaerobic infection.Mater Today Bio. 2024 Aug 10;28:101197. doi: 10.1016/j.mtbio.2024.101197. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39221211 Free PMC article.
-
New Challenges for Anatomists in the Era of Omics.Diagnostics (Basel). 2023 Sep 15;13(18):2963. doi: 10.3390/diagnostics13182963. Diagnostics (Basel). 2023. PMID: 37761332 Free PMC article. Review.
References
-
- Bhattacharjee R. N., Patel S. V. B., Sun Q., Jiang L., Richard-Mohamed M., Ruthirakanthan A., et al. (2020). Renal protection against Ischemia Reperfusion Injury: Hemoglobin-Based Oxygen Carrier-201 versus Blood as an Oxygen Carrier in Ex Vivo Subnormothermic Machine Perfusion. Transplantation 104, 482–489. 10.1097/TP.0000000000002967 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

