Early Initiation of Temozolomide Therapy May Improve Response in Aggressive Pituitary Adenomas
- PMID: 34975752
- PMCID: PMC8718901
- DOI: 10.3389/fendo.2021.774686
Early Initiation of Temozolomide Therapy May Improve Response in Aggressive Pituitary Adenomas
Abstract
Introduction: Aggressive pituitary adenomas (APAs) are, by definition, resistant to optimal multimodality therapy. The challenge lies in their early recognition and timely management. Temozolomide is increasingly being used in patients with APAs, but evidence supporting a favorable response with early initiation is lacking.
Methods: This was a single-center study of all patients with APAs who received at least 3 cycles of temozolomide (150-200 mg/m2). Their baseline clinico-biochemical and radiological profiles were recorded. Immunohistochemical evaluation for cell-cycle markers O6-methylguanine-DNA methyltransferase (MGMT), MutS homolog 2 (MSH2), MutS homolog 6 (MSH6), MutL homolog 1 (MLH1), and postmeiotic segregation increased 2 (PMS2) was performed, and h-scores (product of the number of positive cells and staining intensity) were calculated. Response was assessed in terms of radiological response using the RECIST criteria. Patients with controlled disease (≥30% reduction in tumor volume) were classified as responders.
Results: The study comprised 35 patients (48.6% acromegaly, 37.1% prolactinomas, and 14.3% non-functioning pituitary adenomas). The median number of temozolomide (TMZ) cycles was 9 (IQR 6-14). Responders constituted 68.6% of the cohort and were more likely to have functional tumors, a lower percentage of MGMT-positive staining cells, and lower MGMT h-scores. There was a significantly longer lag period in the initiation of TMZ therapy in non-responders as compared with responders (median 36 vs. 15 months, p = 0.01). ROC-derived cutoffs of 31 months for the duration between diagnosis and TMZ initiation, low-to-intermediate MGMT positivity (40% tumor cells), and MGMT h-score of 80 all had a sensitivity exceeding 80% and a specificity exceeding 70% to predict response.
Conclusion: Early initiation of TMZ therapy, functional tumors, and low MGMT h-score predict a favorable response to TMZ in APAs.
Keywords: MGMT; aggressive pituitary adenomas; controlled disease; early therapy; temozolomide.
Copyright © 2021 Das, Gupta, Dutta, Walia, Vaiphei, Rai, Radotra, Gupta, Sreedharanunni, Ahuja, Bhansali, Tripathi, Sood and Dhandapani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
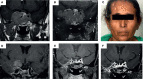
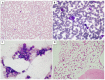
Figures

Similar articles
-
Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide.J Clin Endocrinol Metab. 2015 Apr;100(4):1689-98. doi: 10.1210/jc.2014-4350. Epub 2015 Feb 3. J Clin Endocrinol Metab. 2015. PMID: 25646794
-
Temozolomide responsiveness in aggressive corticotroph tumours: a case report and review of the literature.Pituitary. 2012 Sep;15(3):276-87. doi: 10.1007/s11102-011-0363-7. Pituitary. 2012. PMID: 22076588 Review.
-
MGMT and MSH6 immunoexpression for functioning pituitary macroadenomas.Pituitary. 2017 Dec;20(6):643-653. doi: 10.1007/s11102-017-0829-3. Pituitary. 2017. PMID: 28900805 Free PMC article.
-
Long-course temozolomide in aggressive pituitary adenoma: real-life experience in two tertiary care centers and review of the literature.Pituitary. 2020 Aug;23(4):359-366. doi: 10.1007/s11102-020-01040-4. Pituitary. 2020. PMID: 32232709 Review.
-
DNA mismatch repair protein (MSH6) correlated with the responses of atypical pituitary adenomas and pituitary carcinomas to temozolomide: the national cooperative study by the Japan Society for Hypothalamic and Pituitary Tumors.J Clin Endocrinol Metab. 2013 Mar;98(3):1130-6. doi: 10.1210/jc.2012-2924. Epub 2013 Jan 30. J Clin Endocrinol Metab. 2013. PMID: 23365123
Cited by
-
The biological behavior and clinical outcome of pituitary adenoma are affected by the microenvironment.CNS Neurosci Ther. 2024 May;30(5):e14729. doi: 10.1111/cns.14729. CNS Neurosci Ther. 2024. PMID: 38738958 Free PMC article. Review.
-
Medical treatment of functional pituitary adenomas, trials and tribulations.J Neurooncol. 2024 Jun;168(2):197-213. doi: 10.1007/s11060-024-04670-x. Epub 2024 May 18. J Neurooncol. 2024. PMID: 38760632 Review.
-
Temozolomide Nonresponsiveness in Aggressive Prolactinomas and Carcinomas: Management and Outcomes.J Endocr Soc. 2021 Dec 22;6(2):bvab190. doi: 10.1210/jendso/bvab190. eCollection 2022 Feb 1. J Endocr Soc. 2021. PMID: 35059545 Free PMC article.
-
Prolactin-secreting pituitary adenomas: male-specific differences in pathogenesis, clinical presentation and treatment.Front Endocrinol (Lausanne). 2024 Feb 2;15:1338345. doi: 10.3389/fendo.2024.1338345. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38370355 Free PMC article. Review.
-
Epigenome-wide association study of systemic effects of obesity susceptibility in human twins.Epigenetics. 2023 Dec;18(1):2268834. doi: 10.1080/15592294.2023.2268834. Epub 2023 Oct 23. Epigenetics. 2023. PMID: 37871278 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

