Conjugate of ibrutinib with a TLR7 agonist suppresses melanoma progression and enhances antitumor immunity
- PMID: 34975325
- PMCID: PMC8692160
- DOI: 10.7150/ijbs.64094
Conjugate of ibrutinib with a TLR7 agonist suppresses melanoma progression and enhances antitumor immunity
Abstract
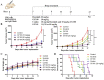
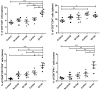
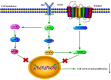
The use of large molecules for immunotherapy has led to exciting developments in cancer treatment, such as the development of PD-1/PD-L1 antibodies. However, small molecule targeted therapies still lack effective immune-functional classes. Ideal anticancer drugs should simultaneously generate immune memory when killing cancer cells to prevent tumor relapse and metastasis. To this end, we carried out a rationally designed strategy to develop novel classes of small molecule compounds with bifunctional targeting and immunostimulatory abilities by conjugating targeting compounds with TLR7 agonists, generating immune-targeting conjugates (ImmunTacs). GY161, as a representative ImmunTac, was synthesized via chemical conjugation of ibrutinib with a TLR7 agonist. In vitro, GY161 stimulated the production of cytokines by mouse spleen lymphocytes, promoted the maturation of dendritic cells (DCs), and inhibited the growth and induced the apoptosis of B16 melanoma cells by regulating the c-Met/β-catenin pathway. In vivo, GY161 enhanced the frequency of CD8+ T cells in spleens and tumors, suppressed the growth of B16 melanoma cell-derived tumors and prolonged the survival time of mice. In summary, GY161 could prevent melanoma progression through direct tumor killing and by triggering specific immunity. These results strongly suggest that ImmunTacs are a reliable and promising strategy for developing small molecule immunogenic anticancer drugs.
Keywords: TLR7 agonist; ibrutinib; immunotherapy; melanoma.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
A chemical conjugation of JQ-1 and a TLR7 agonist induces tumoricidal effects in a murine model of melanoma via enhanced immunomodulation.Int J Cancer. 2021 Jan 15;148(2):437-447. doi: 10.1002/ijc.33222. Epub 2020 Sep 21. Int J Cancer. 2021. PMID: 32683685
-
Additive melanoma suppression with intralesional phospholipid-conjugated TLR7 agonists and systemic IL-2.Melanoma Res. 2011 Feb;21(1):66-75. doi: 10.1097/CMR.0b013e328340ce6c. Melanoma Res. 2011. PMID: 21030882 Free PMC article.
-
Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies.J Immunother Cancer. 2019 Sep 11;7(1):244. doi: 10.1186/s40425-019-0724-8. J Immunother Cancer. 2019. PMID: 31511088 Free PMC article.
-
Self-assembly nanovaccine containing TLR7/8 agonist and STAT3 inhibitor enhances tumor immunotherapy by augmenting tumor-specific immune response.J Immunother Cancer. 2021 Aug;9(8):e003132. doi: 10.1136/jitc-2021-003132. J Immunother Cancer. 2021. PMID: 34452929 Free PMC article.
-
Functional bionanomaterials for cell surface engineering in cancer immunotherapy.APL Bioeng. 2021 May 3;5(2):021506. doi: 10.1063/5.0045945. eCollection 2021 Jun. APL Bioeng. 2021. PMID: 33981940 Free PMC article. Review.
Cited by
-
Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation.Int J Mol Sci. 2024 Jan 4;25(1):663. doi: 10.3390/ijms25010663. Int J Mol Sci. 2024. PMID: 38203835 Free PMC article.
-
Small molecule-based immunomodulators for cancer therapy.Acta Pharm Sin B. 2022 Dec;12(12):4287-4308. doi: 10.1016/j.apsb.2022.11.007. Epub 2022 Nov 12. Acta Pharm Sin B. 2022. PMID: 36562003 Free PMC article. Review.
-
Ibrutinib Modulates Proliferation, Migration, Mitochondrial Homeostasis, and Apoptosis in Melanoma Cells.Biomedicines. 2024 May 4;12(5):1012. doi: 10.3390/biomedicines12051012. Biomedicines. 2024. PMID: 38790974 Free PMC article.
-
The Current State of Treatment and Future Directions in Cutaneous Malignant Melanoma.Biomedicines. 2022 Mar 31;10(4):822. doi: 10.3390/biomedicines10040822. Biomedicines. 2022. PMID: 35453572 Free PMC article. Review.
-
Construction of immunotherapy-related prognostic gene signature and small molecule drug prediction for cutaneous melanoma.Front Oncol. 2022 Jul 25;12:939385. doi: 10.3389/fonc.2022.939385. eCollection 2022. Front Oncol. 2022. PMID: 35957907 Free PMC article.
References
-
- Varikuti S, Singh B, Volpedo G, Ahirwar DK, Jha BK, Saljoughian N, Viana AG, Verma C, Hamza O, Halsey G, Holcomb EA, Maryala RJ, Oghumu S, Ganju RK, Satoskar AR. Ibrutinib treatment inhibits breast cancer progression and metastasis by inducing conversion of myeloid-derived suppressor cells to dendritic cells. Br J Cancer. 2020;122:1005–1013. - PMC - PubMed
-
- Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, Gorjestani S, Liudahl SM, Truitt M, Olson P, Kim G, Hanahan D, Tempero MA, Sheppard B, Irving B. Chang BY, Varner JA, Coussens LM. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 2016;6:270–285. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

