Immunotherapy in Ovarian Cancer: Thinking Beyond PD-1/PD-L1
- PMID: 34966689
- PMCID: PMC8710491
- DOI: 10.3389/fonc.2021.795547
Immunotherapy in Ovarian Cancer: Thinking Beyond PD-1/PD-L1
Abstract
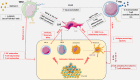
Ovarian cancer (OC) is the most lethal gynecologic malignancy, affecting approximately 1 in 70 women with only 45% surviving 5 years after diagnosis. This disease typically presents at an advanced stage, and optimal debulking with platinum-based chemotherapy remains the cornerstone of management. Although most ovarian cancer patients will respond effectively to current management, 70% of them will eventually develop recurrence and novel therapeutic strategies are needed. There is a rationale for immune-oncological treatments (IO) in the managements of patients with OC. Many OC tumors demonstrate tumor infiltrating lymphocytes (TILs) and the degree of TIL infiltration is strongly and reproducibly correlated with survival. Unfortunately, results to date have been disappointing in relapsed OC. Trials have reported very modest single activity with various antibodies targeting PD-1 or PD-L1 resulting in response rate ranging from 4% to 15%. This may be due to the highly immunosuppressive TME of the disease, a low tumor mutational burden and low PD-L1 expression. There is an urgent need to improve our understanding of the immune microenvironment in OC in order to develop effective therapies. This review will discuss immune subpopulations in OC microenvironment, current immunotherapy modalities targeting these immune subsets and data from clinical trials testing IO treatments in OC and its combination with other therapeutic agents.
Keywords: PD-L1; immunosuppression; immunotherapy; ovarian cancer; tumor microenvironment.
Copyright © 2021 Chardin and Leary.
Conflict of interest statement
AL: fees to institution for ad boards with AZ, Clovis, MSD, GSK, Ability, Merck Serono. AL: support to institution for clinical trials from Roche, GSK, AZ, Agenus, Iovance, Pfizer, MSD, Incyte. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Targeting the immune microenvironment for ovarian cancer therapy.Front Immunol. 2023 Dec 18;14:1328651. doi: 10.3389/fimmu.2023.1328651. eCollection 2023. Front Immunol. 2023. PMID: 38164130 Free PMC article. Review.
-
Current Understanding on Why Ovarian Cancer Is Resistant to Immune Checkpoint Inhibitors.Int J Mol Sci. 2023 Jun 29;24(13):10859. doi: 10.3390/ijms241310859. Int J Mol Sci. 2023. PMID: 37446039 Free PMC article. Review.
-
Strategies to synergize PD-1/PD-L1 targeted cancer immunotherapies to enhance antitumor responses in ovarian cancer.Biochem Pharmacol. 2023 Sep;215:115724. doi: 10.1016/j.bcp.2023.115724. Epub 2023 Jul 29. Biochem Pharmacol. 2023. PMID: 37524205 Review.
-
Immune checkpoint inhibitors in ovarian cancer: where do we stand?Ther Adv Med Oncol. 2021 Aug 18;13:17588359211039899. doi: 10.1177/17588359211039899. eCollection 2021. Ther Adv Med Oncol. 2021. PMID: 34422119 Free PMC article. Review.
-
Massive PD-L1 and CD8 double positive TILs characterize an immunosuppressive microenvironment with high mutational burden in lung cancer.J Immunother Cancer. 2021 Jun;9(6):e002356. doi: 10.1136/jitc-2021-002356. J Immunother Cancer. 2021. PMID: 34140315 Free PMC article.
Cited by
-
Computed Tomography Imaging-Based Radiogenomics Analysis Reveals Hypoxia Patterns and Immunological Characteristics in Ovarian Cancer.Front Immunol. 2022 Mar 28;13:868067. doi: 10.3389/fimmu.2022.868067. eCollection 2022. Front Immunol. 2022. PMID: 35418998 Free PMC article.
-
Profiling the immune landscape in mucinous ovarian carcinoma.Gynecol Oncol. 2023 Jan;168:23-31. doi: 10.1016/j.ygyno.2022.10.022. Epub 2022 Nov 8. Gynecol Oncol. 2023. PMID: 36368129 Free PMC article.
-
Cyclooxygenase-2 Blockade Is Crucial to Restore Natural Killer Cell Activity before Anti-CTLA-4 Therapy against High-Grade Serous Ovarian Cancer.Cancers (Basel). 2023 Dec 22;16(1):80. doi: 10.3390/cancers16010080. Cancers (Basel). 2023. PMID: 38201508 Free PMC article.
-
Effectiveness and safety of nab-paclitaxel and platinum as first-line chemotherapy for ovarian cancer: a retrospective study.J Gynecol Oncol. 2023 Jul;34(4):e44. doi: 10.3802/jgo.2023.34.e44. Epub 2023 Feb 6. J Gynecol Oncol. 2023. PMID: 36807747 Free PMC article.
-
Classifying cGAS-STING Activity Links Chromosomal Instability with Immunotherapy Response in Metastatic Bladder Cancer.Cancer Res Commun. 2022 Aug 4;2(8):762-771. doi: 10.1158/2767-9764.CRC-22-0047. eCollection 2022 Aug. Cancer Res Commun. 2022. PMID: 36923311 Free PMC article.
References
-
- Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. . Primary Chemotherapy Versus Primary Surgery for Newly Diagnosed Advanced Ovarian Cancer (CHORUS): An Open-Label, Randomised, Controlled, Non-Inferiority Trial. Lancet Lond Engl (2015) 386:249–57. doi: 10.1016/S0140-6736(14)62223-6 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

