Biological Functions and Regulatory Mechanisms of Hypoxia-Inducible Factor-1α in Ischemic Stroke
- PMID: 34966392
- PMCID: PMC8710457
- DOI: 10.3389/fimmu.2021.801985
Biological Functions and Regulatory Mechanisms of Hypoxia-Inducible Factor-1α in Ischemic Stroke
Abstract
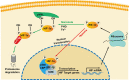
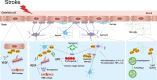
Ischemic stroke is caused by insufficient cerebrovascular blood and oxygen supply. It is a major contributor to death or disability worldwide and has become a heavy societal and clinical burden. To date, effective treatments for ischemic stroke are limited, and innovative therapeutic methods are urgently needed. Hypoxia inducible factor-1α (HIF-1α) is a sensitive regulator of oxygen homeostasis, and its expression is rapidly induced after hypoxia/ischemia. It plays an extensive role in the pathophysiology of stroke, including neuronal survival, neuroinflammation, angiogenesis, glucose metabolism, and blood brain barrier regulation. In addition, the spatiotemporal expression profile of HIF-1α in the brain shifts with the progression of ischemic stroke; this has led to contradictory findings regarding its function in previous studies. Therefore, unveiling the Janus face of HIF-1α and its target genes in different type of cells and exploring the role of HIF-1α in inflammatory responses after ischemia is of great importance for revealing the pathogenesis and identifying new therapeutic targets for ischemic stroke. Herein, we provide a succinct overview of the current approaches targeting HIF-1α and summarize novel findings concerning HIF-1α regulation in different types of cells within neurovascular units, including neurons, endothelial cells, astrocytes, and microglia, during the different stages of ischemic stroke. The current representative translational approaches focused on neuroprotection by targeting HIF-1α are also discussed.
Keywords: HIF-1α; hypoxia; ischemic stroke; neuroinflammation; neuroprotection; neurovascular unit.
Copyright © 2021 He, Ma, Liu, Zhang, Ren, Zhao, Chang, Guo and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
HIF-1, an important regulator in potential new therapeutic approaches to ischemic stroke.Neurochem Int. 2023 Nov;170:105605. doi: 10.1016/j.neuint.2023.105605. Epub 2023 Aug 30. Neurochem Int. 2023. PMID: 37657765 Review.
-
The Janus face of HIF-1α in ischemic stroke and the possible associated pathways.Neurochem Int. 2024 Jul;177:105747. doi: 10.1016/j.neuint.2024.105747. Epub 2024 Apr 22. Neurochem Int. 2024. PMID: 38657682 Review.
-
Role of NADPH Oxidase-Induced Hypoxia-Induced Factor-1α Increase in Blood-Brain Barrier Disruption after 2-Hour Focal Ischemic Stroke in Rat.Neural Plast. 2021 Aug 14;2021:9928232. doi: 10.1155/2021/9928232. eCollection 2021. Neural Plast. 2021. PMID: 34434231 Free PMC article.
-
Hypoxia Inducible Factor 1α Plays a Key Role in Remote Ischemic Preconditioning Against Stroke by Modulating Inflammatory Responses in Rats.J Am Heart Assoc. 2018 Feb 24;7(5):e007589. doi: 10.1161/JAHA.117.007589. J Am Heart Assoc. 2018. PMID: 29478025 Free PMC article.
-
Hypoxia Inducible Factor-1α Attenuates Ischemic Brain Damage by Modulating Inflammatory Response and Glial Activity.Cells. 2021 Jun 1;10(6):1359. doi: 10.3390/cells10061359. Cells. 2021. PMID: 34205911 Free PMC article.
Cited by
-
Potential role of serum hypoxia-inducible factor 1alpha as a biomarker of delayed cerebral ischemia and poor clinical outcome after human aneurysmal subarachnoid hemorrhage: A prospective, longitudinal, multicenter, and observational study.Front Neurol. 2022 Dec 8;13:1072351. doi: 10.3389/fneur.2022.1072351. eCollection 2022. Front Neurol. 2022. PMID: 36570456 Free PMC article.
-
Key roles of glial cells in the encephalopathy of prematurity.Glia. 2024 Mar;72(3):475-503. doi: 10.1002/glia.24474. Epub 2023 Nov 1. Glia. 2024. PMID: 37909340 Free PMC article. Review.
-
Phagocytic microglia and macrophages in brain injury and repair.CNS Neurosci Ther. 2022 Sep;28(9):1279-1293. doi: 10.1111/cns.13899. Epub 2022 Jun 25. CNS Neurosci Ther. 2022. PMID: 35751629 Free PMC article. Review.
-
Role of hypoxia inducible factor HIF-1α in heart valves.Glob Cardiol Sci Pract. 2023 May 11;2023(2):e202309. doi: 10.21542/gcsp.2023.9. eCollection 2023 May 11. Glob Cardiol Sci Pract. 2023. PMID: 37351095 Free PMC article. Review.
-
Prototheca bovis induces autophagy in bovine mammary epithelial cells via the HIF-1α and AMPKα/ULK1 pathway.Front Immunol. 2022 Sep 2;13:934819. doi: 10.3389/fimmu.2022.934819. eCollection 2022. Front Immunol. 2022. PMID: 36148236 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

