Local Treatment of a Pediatric Osteosarcoma Model with a 4-1BBL Armed Oncolytic Adenovirus Results in an Antitumor Effect and Leads to Immune Memory
- PMID: 34965961
- PMCID: PMC7612474
- DOI: 10.1158/1535-7163.MCT-21-0565
Local Treatment of a Pediatric Osteosarcoma Model with a 4-1BBL Armed Oncolytic Adenovirus Results in an Antitumor Effect and Leads to Immune Memory
Abstract
Osteosarcoma is an aggressive bone tumor occurring primarily in pediatric patients. Despite years of intensive research, the outcomes of patients with metastatic disease or those who do not respond to therapy have remained poor and have not changed in the last 30 years. Oncolytic virotherapy is becoming a reality to treat local and metastatic tumors while maintaining a favorable safety profile. Delta-24-ACT is a replicative oncolytic adenovirus engineered to selectively target cancer cells and to potentiate immune responses through expression of the immune costimulatory ligand 4-1BB. This work aimed to assess the antisarcoma effect of Delta-24-ACT. MTS and replication assays were used to quantify the antitumor effects of Delta-24-ACT in vitro in osteosarcoma human and murine cell lines. Evaluation of the in vivo antitumor effect and immune response to Delta-24-ACT was performed in immunocompetent mice bearing the orthotopic K7M2 cell line. Immunophenotyping of the tumor microenvironment was characterized by immunohistochemistry and flow cytometry. In vitro, Delta-24-ACT killed osteosarcoma cells and triggered the production of danger signals. In vivo, local treatment with Delta-24-ACT led to antitumor effects against both the primary tumor and spontaneous metastases in a murine osteosarcoma model. Viral treatment was safe, with no noted toxicity. Delta-24-ACT significantly increased the median survival time of treated mice. Collectively, our data identify Delta-24-ACT administration as an effective and safe therapeutic strategy for patients with local and metastatic osteosarcoma. These results support clinical translation of this viral immunotherapy approach.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures
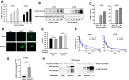
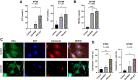
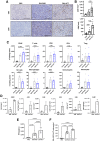
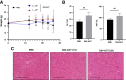

Comment in
- Mol Cancer Ther. 21:395.
- Mol Cancer Ther. 21:395.
Similar articles
-
Oncolytic Viral Therapy in Osteosarcoma.Viruses. 2024 Jul 16;16(7):1139. doi: 10.3390/v16071139. Viruses. 2024. PMID: 39066301 Free PMC article. Review.
-
The Oncolytic Adenovirus VCN-01 as Therapeutic Approach Against Pediatric Osteosarcoma.Clin Cancer Res. 2016 May 1;22(9):2217-25. doi: 10.1158/1078-0432.CCR-15-1899. Epub 2015 Nov 24. Clin Cancer Res. 2016. PMID: 26603261
-
Combination immunotherapy using G-CSF and oncolytic virotherapy reduces tumor growth in osteosarcoma.J Immunother Cancer. 2021 Mar;9(3):e001703. doi: 10.1136/jitc-2020-001703. J Immunother Cancer. 2021. PMID: 33737338 Free PMC article.
-
Intravenous administration of the conditionally replicative adenovirus Ad5-Delta24RGD induces regression of osteosarcoma lung metastases.Mol Cancer. 2008 Jan 23;7:9. doi: 10.1186/1476-4598-7-9. Mol Cancer. 2008. PMID: 18215325 Free PMC article.
-
Oncolytic viruses for potential osteosarcoma therapy.Adv Exp Med Biol. 2014;804:259-83. doi: 10.1007/978-3-319-04843-7_14. Adv Exp Med Biol. 2014. PMID: 24924179 Review.
Cited by
-
Galectin-3 inhibition boosts the therapeutic efficacy of Semliki Forest virus in pediatric osteosarcoma.Mol Ther Oncolytics. 2022 Jul 9;26:246-264. doi: 10.1016/j.omto.2022.07.004. eCollection 2022 Sep 15. Mol Ther Oncolytics. 2022. PMID: 35949950 Free PMC article.
-
p53-armed oncolytic virotherapy induces abscopal effect in osteosarcoma by promoting immunogenic cell death.Mol Ther Oncol. 2024 Jun 29;32(3):200845. doi: 10.1016/j.omton.2024.200845. eCollection 2024 Sep 19. Mol Ther Oncol. 2024. PMID: 39108499 Free PMC article.
-
Immunovirotherapy for Pediatric Solid Tumors: A Promising Treatment That is Becoming a Reality.Front Immunol. 2022 Apr 13;13:866892. doi: 10.3389/fimmu.2022.866892. eCollection 2022. Front Immunol. 2022. PMID: 35493490 Free PMC article. Review.
-
Oncolytic Viral Therapy in Osteosarcoma.Viruses. 2024 Jul 16;16(7):1139. doi: 10.3390/v16071139. Viruses. 2024. PMID: 39066301 Free PMC article. Review.
-
Personalizing Oncolytic Immunovirotherapy Approaches.Mol Diagn Ther. 2024 Mar;28(2):153-168. doi: 10.1007/s40291-023-00689-4. Epub 2023 Dec 27. Mol Diagn Ther. 2024. PMID: 38150172
References
-
- Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther 2006;6:1075–85. - PubMed
-
- Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. . Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst 2003;95:652–60. - PubMed
-
- Suzuki K, Alemany R, Yamamoto M, Curie DT. The presence of the adenovirus E3 region improves the oncolytic potency of conditionally replicative adenoviruses. Clin Cancer Res 2002;8:3348–59. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

