Alzheimer's vulnerable brain region relies on a distinct retromer core dedicated to endosomal recycling
- PMID: 34965419
- PMCID: PMC8792909
- DOI: 10.1016/j.celrep.2021.110182
Alzheimer's vulnerable brain region relies on a distinct retromer core dedicated to endosomal recycling
Abstract
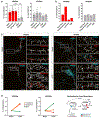
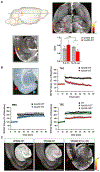
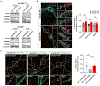
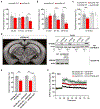
Whether and how the pathogenic disruptions in endosomal trafficking observed in Alzheimer's disease (AD) are linked to its anatomical vulnerability remain unknown. Here, we began addressing these questions by showing that neurons are enriched with a second retromer core, organized around VPS26b, differentially dedicated to endosomal recycling. Next, by imaging mouse models, we show that the trans-entorhinal cortex, a region most vulnerable to AD, is most susceptible to VPS26b depletion-a finding validated by electrophysiology, immunocytochemistry, and behavior. VPS26b was then found enriched in the trans-entorhinal cortex of human brains, where both VPS26b and the retromer-related receptor SORL1 were found deficient in AD. Finally, by regulating glutamate receptor and SORL1 recycling, we show that VPS26b can mediate regionally selective synaptic dysfunction and SORL1 deficiency. Together with the trans-entorhinal's unique network properties, hypothesized to impose a heavy demand on endosomal recycling, these results suggest a general mechanism that can explain AD's regional vulnerability.
Keywords: Alzheimer’s disease; VPS26b; endosomal trafficking; retromer; trans-entorhinal cortex.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S.A.S. is a co-founder of Retromer Therapeutics, has equity in the company, and is a paid consultant to the company. In addition, S.A.S. has equity in Imij Technologies, an MRI-based company. G.D.P. is a full-time employee of Denali Therapeutics, Inc. O.M.A. has commercial interests in Retromer Therapeutics. Lastly, S.A.S., S.S., and Y.H.Q. are listed as co-inventors on Columbia University-owned patents that relate to retromer biomarkers and retromer drug discovery targets.
Figures


Similar articles
-
Pharmacologic enhancement of retromer rescues endosomal pathology induced by defects in the Alzheimer's gene SORL1.Stem Cell Reports. 2023 Dec 12;18(12):2434-2450. doi: 10.1016/j.stemcr.2023.10.011. Epub 2023 Nov 9. Stem Cell Reports. 2023. PMID: 37949073 Free PMC article.
-
The Alzheimer's gene SORL1 is a regulator of endosomal traffic and recycling in human neurons.Cell Mol Life Sci. 2022 Feb 28;79(3):162. doi: 10.1007/s00018-022-04182-9. Cell Mol Life Sci. 2022. PMID: 35226190 Free PMC article.
-
Depletion of the AD Risk Gene SORL1 Selectively Impairs Neuronal Endosomal Traffic Independent of Amyloidogenic APP Processing.Cell Rep. 2020 Jun 2;31(9):107719. doi: 10.1016/j.celrep.2020.107719. Cell Rep. 2020. PMID: 32492427 Free PMC article.
-
Sorting receptor SORLA--a trafficking path to avoid Alzheimer disease.J Cell Sci. 2013 Jul 1;126(Pt 13):2751-60. doi: 10.1242/jcs.125393. Epub 2013 Jun 26. J Cell Sci. 2013. PMID: 23813966 Review.
-
Differential effects of SORL1 deficiency on the endo-lysosomal network in human neurons and microglia.Philos Trans R Soc Lond B Biol Sci. 2024 Apr 8;379(1899):20220389. doi: 10.1098/rstb.2022.0389. Epub 2024 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38368935 Free PMC article. Review.
Cited by
-
A genetically modified minipig model for Alzheimer's disease with SORL1 haploinsufficiency.Cell Rep Med. 2022 Sep 20;3(9):100740. doi: 10.1016/j.xcrm.2022.100740. Epub 2022 Sep 12. Cell Rep Med. 2022. PMID: 36099918 Free PMC article.
-
A familial missense variant in the Alzheimer's disease gene SORL1 impairs its maturation and endosomal sorting.Acta Neuropathol. 2024 Jan 20;147(1):20. doi: 10.1007/s00401-023-02670-1. Acta Neuropathol. 2024. PMID: 38244079 Free PMC article.
-
Finding memo: versatile interactions of the VPS10p-Domain receptors in Alzheimer's disease.Mol Neurodegener. 2022 Nov 18;17(1):74. doi: 10.1186/s13024-022-00576-2. Mol Neurodegener. 2022. PMID: 36397124 Free PMC article. Review.
-
TDP-43-stratified single-cell proteomics of postmortem human spinal motor neurons reveals protein dynamics in amyotrophic lateral sclerosis.Cell Rep. 2024 Jan 23;43(1):113636. doi: 10.1016/j.celrep.2023.113636. Epub 2024 Jan 5. Cell Rep. 2024. PMID: 38183652 Free PMC article.
-
Beware of Misdelivery: Multifaceted Role of Retromer Transport in Neurodegenerative Diseases.Front Aging Neurosci. 2022 May 6;14:897688. doi: 10.3389/fnagi.2022.897688. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35601613 Free PMC article. Review.
References
-
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, and Morgan D (2006). Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat. Protoc 1, 1671–1679. - PubMed
-
- Andersen OM, Bogh N, Landua AM, Ploen GG, Jensen AM, Monti G, Ulhon BP, Nyengaard JR, Jacobsen K, Jorgensen M, et al. (2021). In vivo evidence that SORL1, encoding the endosomal recycling receptor SORLA, can function as a casual gene in Alzheimer’s Disease. bioRxiv. 10.1101/2021.07.13.452149. - DOI
-
- Angulo SL, Henzi T, Neymotin SA, Suarez MD, Lytton WW, Schwaller B, and Moreno H (2020). Amyloid pathology-produced unexpected modifications of calcium homeostasis in hippocampal subicular dendrites 16, 251–261. - PubMed
-
- Attar N, and Cullen PJ (2010). The retromer complex. Adv. Enzyme Regul 50,216–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

