Decellularized extracellular matrix mediates tissue construction and regeneration
- PMID: 34962624
- PMCID: PMC8976706
- DOI: 10.1007/s11684-021-0900-3
Decellularized extracellular matrix mediates tissue construction and regeneration
Abstract
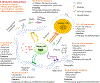
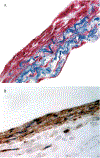
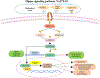
Contributing to organ formation and tissue regeneration, extracellular matrix (ECM) constituents provide tissue with three-dimensional (3D) structural integrity and cellular-function regulation. Containing the crucial traits of the cellular microenvironment, ECM substitutes mediate cell-matrix interactions to prompt stem-cell proliferation and differentiation for 3D organoid construction in vitro or tissue regeneration in vivo. However, these ECMs are often applied generically and have yet to be extensively developed for specific cell types in 3D cultures. Cultured cells also produce rich ECM, particularly stromal cells. Cellular ECM improves 3D culture development in vitro and tissue remodeling during wound healing after implantation into the host as well. Gaining better insight into ECM derived from either tissue or cells that regulate 3D tissue reconstruction or organ regeneration helps us to select, produce, and implant the most suitable ECM and thus promote 3D organoid culture and tissue remodeling for in vivo regeneration. Overall, the decellularization methodologies and tissue/cell-derived ECM as scaffolds or cellular-growth supplements used in cell propagation and differentiation for 3D tissue culture in vitro are discussed. Moreover, current preclinical applications by which ECM components modulate the wound-healing process are reviewed.
Keywords: 3D culture; decellularized extracellular matrix; organoids; tissue repair.
© 2021. The Author(s).
Conflict of interest statement
Compliance with ethics guidelines
Chuanqi Liu, Ming Pei, Qingfeng Li, and Yuanyuan Zhang declare that they have no conflict of interest. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
Figures

Similar articles
-
Decellularized extracellular matrix-decorated 3D nanofiber scaffolds enhance cellular responses and tissue regeneration.Acta Biomater. 2024 Aug;184:81-97. doi: 10.1016/j.actbio.2024.06.020. Epub 2024 Jun 21. Acta Biomater. 2024. PMID: 38908416
-
Cell type-specific extracellular matrix guided the differentiation of human mesenchymal stem cells in 3D polymeric scaffolds.J Mater Sci Mater Med. 2017 Jul;28(7):100. doi: 10.1007/s10856-017-5912-9. Epub 2017 May 22. J Mater Sci Mater Med. 2017. PMID: 28534283 Free PMC article.
-
Extracellular matrix derived by human umbilical cord-deposited mesenchymal stem cells accelerates chondrocyte proliferation and differentiation potential in vitro.Cell Tissue Bank. 2019 Sep;20(3):351-365. doi: 10.1007/s10561-019-09774-7. Epub 2019 Jun 19. Cell Tissue Bank. 2019. PMID: 31218457
-
Enhancing organoid culture: harnessing the potential of decellularized extracellular matrix hydrogels for mimicking microenvironments.J Biomed Sci. 2024 Sep 27;31(1):96. doi: 10.1186/s12929-024-01086-7. J Biomed Sci. 2024. PMID: 39334251 Free PMC article. Review.
-
Recent advancements in decellularized matrix technology for bone tissue engineering.Differentiation. 2021 Sep-Oct;121:25-34. doi: 10.1016/j.diff.2021.08.004. Epub 2021 Aug 23. Differentiation. 2021. PMID: 34454348 Review.
Cited by
-
Mechanism and application of fibrous proteins in diabetic wound healing: a literature review.Front Endocrinol (Lausanne). 2024 Jul 26;15:1430543. doi: 10.3389/fendo.2024.1430543. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39129915 Free PMC article. Review.
-
Regenerative Potential of A Bovine ECM-Derived Hydrogel for Biomedical Applications.Biomolecules. 2022 Sep 2;12(9):1222. doi: 10.3390/biom12091222. Biomolecules. 2022. PMID: 36139063 Free PMC article.
-
Functional acellular matrix for tissue repair.Mater Today Bio. 2022 Dec 28;18:100530. doi: 10.1016/j.mtbio.2022.100530. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36601535 Free PMC article. Review.
-
Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration.Biomolecules. 2023 Apr 14;13(4):673. doi: 10.3390/biom13040673. Biomolecules. 2023. PMID: 37189420 Free PMC article. Review.
-
Bioinks of Natural Biomaterials for Printing Tissues.Bioengineering (Basel). 2023 Jun 10;10(6):705. doi: 10.3390/bioengineering10060705. Bioengineering (Basel). 2023. PMID: 37370636 Free PMC article.
References
-
- Prewitz MC, Seib FP, von Bonin M, Friedrichs J, Stißel A, Niehage C, Müller K, Anastassiadis K, Waskow C, Hoflack B, Bornhäuser M, Werner C. Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods 2013; 10(8): 788–794 - PubMed
-
- Sart S, Jeske R, Chen X, Ma T, Li Y. Engineering stem cell-derived extracellular matrices: decellularization, characterization, and biological function. Tissue Eng Part B Rev 2020; 26(5): 402–422 - PubMed
-
- Sart S, Agathos SN, Li Y. Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol Prog 2013; 29(6): 1354–1366 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

