Improved Anticancer Activities of a New Pentafluorothio-Substituted Vorinostat-Type Histone Deacetylase Inhibitor
- PMID: 34959719
- PMCID: PMC8704709
- DOI: 10.3390/ph14121319
Improved Anticancer Activities of a New Pentafluorothio-Substituted Vorinostat-Type Histone Deacetylase Inhibitor
Abstract
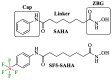
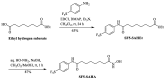
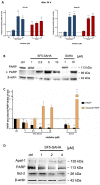
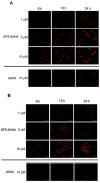
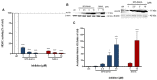
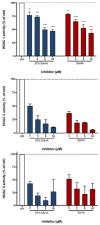
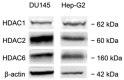
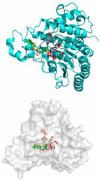
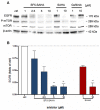
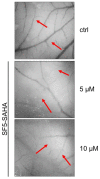
The development of new anticancer drugs is necessary in order deal with the disease and with the drawbacks of currently applied drugs. Epigenetic dysregulations are a central hallmark of cancerogenesis and histone deacetylases (HDACs) emerged as promising anticancer targets. HDAC inhibitors are promising epigenetic anticancer drugs and new HDAC inhibitors are sought for in order to obtain potent drug candidates. The new HDAC inhibitor SF5-SAHA was synthesized and analyzed for its anticancer properties. The new compound SF5-SAHA showed strong inhibition of tumor cell growth with IC50 values similar to or lower than that of the clinically applied reference compound vorinostat/SAHA (suberoylanilide hydroxamic acid). Target specific HDAC inhibition was demonstrated by Western blot analyses. Unspecific cytotoxic effects were not observed in LDH-release measurements. Pro-apoptotic formation of reactive oxygen species (ROS) and caspase-3 activity induction in prostate carcinoma and hepatocellular carcinoma cell lines DU145 and Hep-G2 seem to be further aspects of the mode of action. Antiangiogenic activity of SF5-SAHA was observed on chorioallantoic membranes of fertilized chicken eggs (CAM assay). The presence of the pentafluorothio-substituent of SF5-SAHA increased the antiproliferative effects in both solid tumor and leukemia/lymphoma cell models when compared with its parent compound vorinostat. Based on this preliminary study, SF5-SAHA has the prerequisites to be further developed as a new HDAC inhibitory anticancer drug candidate.
Keywords: anticancer drugs; fluorine; histone deacetylase inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
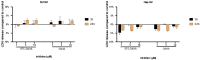


Similar articles
-
Anticancer Activity and Mechanisms of Action of New Chimeric EGFR/HDAC-Inhibitors.Int J Mol Sci. 2021 Aug 5;22(16):8432. doi: 10.3390/ijms22168432. Int J Mol Sci. 2021. PMID: 34445133 Free PMC article.
-
Structural Requirements of Histone Deacetylase Inhibitors: SAHA Analogs Modified on the Hydroxamic Acid.Arch Pharm (Weinheim). 2016 May;349(5):373-82. doi: 10.1002/ardp.201500472. Epub 2016 Apr 9. Arch Pharm (Weinheim). 2016. PMID: 27062198 Free PMC article.
-
Anticancer effects of the MHY218 novel hydroxamic acid-derived histone deacetylase inhibitor in human ovarian cancer cells.Int J Oncol. 2010 Aug;37(2):419-28. doi: 10.3892/ijo_00000690. Int J Oncol. 2010. PMID: 20596669
-
Multimodal HDAC Inhibitors with Improved Anticancer Activity.Curr Cancer Drug Targets. 2018;18(1):39-56. doi: 10.2174/1568009617666170206102613. Curr Cancer Drug Targets. 2018. PMID: 28176653 Review.
-
Vorinostat (SAHA) and Breast Cancer: An Overview.Cancers (Basel). 2021 Sep 19;13(18):4700. doi: 10.3390/cancers13184700. Cancers (Basel). 2021. PMID: 34572928 Free PMC article. Review.
Cited by
-
Modified Suberoylanilide Hydroxamic Acid Reduced Drug-Associated Immune Cell Death and Organ Damage under Lipopolysaccharide Inflammatory Challenge.ACS Pharmacol Transl Sci. 2022 Oct 10;5(11):1128-1141. doi: 10.1021/acsptsci.2c00119. eCollection 2022 Nov 11. ACS Pharmacol Transl Sci. 2022. PMID: 36407956 Free PMC article.
-
The Impact of Fluorination on the Design of Histone Deacetylase Inhibitors.Molecules. 2023 Feb 19;28(4):1973. doi: 10.3390/molecules28041973. Molecules. 2023. PMID: 36838960 Free PMC article. Review.
-
Superior Anticancer and Antifungal Activities of New Sulfanyl-Substituted Niclosamide Derivatives.Biomedicines. 2024 Jul 21;12(7):1621. doi: 10.3390/biomedicines12071621. Biomedicines. 2024. PMID: 39062194 Free PMC article.
-
Molecular Mechanisms in Tumorigenesis of Hepatocellular Carcinoma and in Target Treatments-An Overview.Biomolecules. 2024 Jun 4;14(6):656. doi: 10.3390/biom14060656. Biomolecules. 2024. PMID: 38927059 Free PMC article. Review.
-
Novel Thienyl-Based Tyrosine Kinase Inhibitors for the Treatment of Hepatocellular Carcinoma.J Pers Med. 2022 May 1;12(5):738. doi: 10.3390/jpm12050738. J Pers Med. 2022. PMID: 35629160 Free PMC article.
References
-
- Sippl W., Jung M. Epigenetic Drug Discovery. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

