Antisense Oligonucleotide-Based Therapy of Viral Infections
- PMID: 34959297
- PMCID: PMC8707165
- DOI: 10.3390/pharmaceutics13122015
Antisense Oligonucleotide-Based Therapy of Viral Infections
Abstract
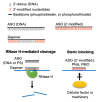
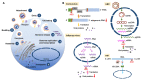
Nucleic acid-based therapeutics have demonstrated their efficacy in the treatment of various diseases and vaccine development. Antisense oligonucleotide (ASO) technology exploits a single-strand short oligonucleotide to either cause target RNA degradation or sterically block the binding of cellular factors or machineries to the target RNA. Chemical modification or bioconjugation of ASOs can enhance both its pharmacokinetic and pharmacodynamic performance, and it enables customization for a specific clinical purpose. ASO-based therapies have been used for treatment of genetic disorders, cancer and viral infections. In particular, ASOs can be rapidly developed for newly emerging virus and their reemerging variants. This review discusses ASO modifications and delivery options as well as the design of antiviral ASOs. A better understanding of the viral life cycle and virus-host interactions as well as advances in oligonucleotide technology will benefit the development of ASO-based antiviral therapies.
Keywords: RNA therapeutics; antisense oligonucleotide; drug delivery; virus; virus-host interaction.
Conflict of interest statement
The authors declare no conflict and interest.
Figures


Similar articles
-
Medicinal Chemistry of Antisense Oligonucleotides for Therapeutic Use in SARS-CoV-2: Design Strategies and Challenges for Targeted Delivery.Curr Med Chem. 2024 Jun 11. doi: 10.2174/0109298673300236240529195835. Online ahead of print. Curr Med Chem. 2024. PMID: 38860908
-
Antisense oligonucleotide-based therapies for the treatment of osteoarthritis: Opportunities and roadblocks.Bone. 2020 Sep;138:115461. doi: 10.1016/j.bone.2020.115461. Epub 2020 May 30. Bone. 2020. PMID: 32485363 Review.
-
Drug Discovery Perspectives of Antisense Oligonucleotides.Biomol Ther (Seoul). 2023 May 1;31(3):241-252. doi: 10.4062/biomolther.2023.001. Epub 2023 Mar 2. Biomol Ther (Seoul). 2023. PMID: 36859811 Free PMC article. Review.
-
Antisense oligonucleotide therapeutics in neurodegenerative diseases: the case of polyglutamine disorders.Brain. 2020 Feb 1;143(2):407-429. doi: 10.1093/brain/awz328. Brain. 2020. PMID: 31738395 Review.
-
Recent Advances in Antisense Oligonucleotide Therapy in Genetic Neuromuscular Diseases.Ann Indian Acad Neurol. 2018 Jan-Mar;21(1):3-8. doi: 10.4103/aian.AIAN_298_17. Ann Indian Acad Neurol. 2018. PMID: 29720791 Free PMC article. Review.
Cited by
-
Supportive Oligonucleotide Therapy (SOT) as a Potential Treatment for Viral Infections and Lyme Disease: Preliminary Results.Infect Dis Rep. 2022 Nov 3;14(6):824-836. doi: 10.3390/idr14060084. Infect Dis Rep. 2022. PMID: 36412742 Free PMC article.
-
Reduction of Tumor Growth with RNA-Targeting Treatment of the NAB2-STAT6 Fusion Transcript in Solitary Fibrous Tumor Models.Cancers (Basel). 2023 Jun 9;15(12):3127. doi: 10.3390/cancers15123127. Cancers (Basel). 2023. PMID: 37370737 Free PMC article.
-
Novel antiviral approaches for Marburg: a promising therapeutics in the pipeline.Front Microbiol. 2024 Apr 25;15:1387628. doi: 10.3389/fmicb.2024.1387628. eCollection 2024. Front Microbiol. 2024. PMID: 38725678 Free PMC article. Review.
-
Effects of combinations of gapmer antisense oligonucleotides on the target reduction.Mol Biol Rep. 2023 Apr;50(4):3539-3546. doi: 10.1007/s11033-022-08224-0. Epub 2023 Feb 14. Mol Biol Rep. 2023. PMID: 36787053 Free PMC article.
-
Non-Coding RNAs in HIV Infection, NeuroHIV, and Related Comorbidities.Cells. 2024 May 23;13(11):898. doi: 10.3390/cells13110898. Cells. 2024. PMID: 38891030 Free PMC article. Review.
References
-
- 10 Threats to Global Health in 2019. [(accessed on 25 October 2019)]. Available online: https://www.who.int.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

