Lymphatic Reconstruction in Kidney Allograft Aggravates Chronic Rejection by Promoting Alloantigen Presentation
- PMID: 34956231
- PMCID: PMC8695730
- DOI: 10.3389/fimmu.2021.796260
Lymphatic Reconstruction in Kidney Allograft Aggravates Chronic Rejection by Promoting Alloantigen Presentation
Erratum in
-
Corrigendum: Lymphatic reconstruction in kidney allograft aggravates chronic rejection by promoting alloantigen presentation.Front Immunol. 2022 Nov 24;13:1071763. doi: 10.3389/fimmu.2022.1071763. eCollection 2022. Front Immunol. 2022. PMID: 36505502 Free PMC article.
Abstract
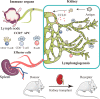
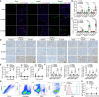
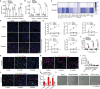
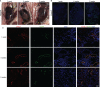
Chronic rejection of the renal allograft remains a major cause of graft loss. Here, we demonstrated that the remodeling of lymphatic vessels (LVs) after their broken during transplantation contributes to the antigen presenting and lymph nodes activating. Our studies observed a rebuilt of interrupted lymph draining one week after mouse kidney transplantation, involving preexisting lymphatic endothelial cells (LECs) from both the donor and recipient. These expanding LVs also release C-C chemokine ligand 21 (CCL21) and recruit CCR7+ cells, mainly dendritic cells (DCs), toward lymph nodes and spleen, evoking the adaptive response. This rejection could be relieved by LYVE-1 specific LVs knockout or CCR7 migration inhibition in mouse model. Moreover, in retrospective analysis, posttransplant patients exhibiting higher area density of LVs presented with lower eGFR, severe serum creatinine and proteinuria, and greater interstitial fibrosis. These results reveal a rebuilt pathway for alloantigen trafficking and lymphocytes activation, providing strategies to alleviate chronic transplantation rejection.
Keywords: allograft; chronic rejection; inflammation; lymphangiogenesis; renal transplantation.
Copyright © 2021 Lin, Chen, Zhu, Cheng, Wang, Yu, Tang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Chronic Rejection of Cardiac Allografts Is Associated With Increased Lymphatic Flow and Cellular Trafficking.Circulation. 2018 Jan 30;137(5):488-503. doi: 10.1161/CIRCULATIONAHA.117.028533. Epub 2017 Aug 3. Circulation. 2018. PMID: 28775077 Free PMC article.
-
Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts.Circulation. 2010 Mar 30;121(12):1413-22. doi: 10.1161/CIRCULATIONAHA.109.910703. Epub 2010 Mar 15. Circulation. 2010. PMID: 20231530
-
Lymphangiogenesis in kidney and lymph node mediates renal inflammation and fibrosis.Sci Adv. 2019 Jun 26;5(6):eaaw5075. doi: 10.1126/sciadv.aaw5075. eCollection 2019 Jun. Sci Adv. 2019. PMID: 31249871 Free PMC article.
-
B cells as antigen-presenting cells in transplantation rejection and tolerance.Cell Immunol. 2020 Mar;349:104061. doi: 10.1016/j.cellimm.2020.104061. Epub 2020 Feb 7. Cell Immunol. 2020. PMID: 32059816 Free PMC article. Review.
-
Emerging role of exosomes in allorecognition and allograft rejection.Curr Opin Organ Transplant. 2018 Feb;23(1):22-27. doi: 10.1097/MOT.0000000000000489. Curr Opin Organ Transplant. 2018. PMID: 29189413 Free PMC article. Review.
Cited by
-
Transcript Engineered Extracellular Vesicles Alleviate Alloreactive Dynamics in Renal Transplantation.Adv Sci (Weinh). 2022 Nov;9(31):e2202633. doi: 10.1002/advs.202202633. Epub 2022 Sep 8. Adv Sci (Weinh). 2022. PMID: 36073846 Free PMC article.
-
Immune surveillance and humoral immune responses in kidney transplantation - A look back at T follicular helper cells.Front Immunol. 2023 Jul 12;14:1114842. doi: 10.3389/fimmu.2023.1114842. eCollection 2023. Front Immunol. 2023. PMID: 37503334 Free PMC article. Review.
-
Dysregulation of Lymphatic Endothelial VEGFR3 Signaling in Disease.Cells. 2023 Dec 28;13(1):68. doi: 10.3390/cells13010068. Cells. 2023. PMID: 38201272 Free PMC article. Review.
-
Acute kidney injury results in long-term alterations of kidney lymphatics in mice.Am J Physiol Renal Physiol. 2024 Nov 1;327(5):F869-F884. doi: 10.1152/ajprenal.00120.2024. Epub 2024 Sep 26. Am J Physiol Renal Physiol. 2024. PMID: 39323387
-
CCR7 and CD48 as Predicted Targets in Acute Rejection Related to M1 Macrophage after Pediatric Kidney Transplantation.J Immunol Res. 2024 Jun 24;2024:6908968. doi: 10.1155/2024/6908968. eCollection 2024. J Immunol Res. 2024. PMID: 38957433 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

