Early Thalamic Injury After Resuscitation From Severe Asphyxial Cardiac Arrest in Developing Rats
- PMID: 34950655
- PMCID: PMC8688916
- DOI: 10.3389/fcell.2021.737319
Early Thalamic Injury After Resuscitation From Severe Asphyxial Cardiac Arrest in Developing Rats
Abstract
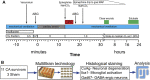
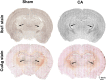
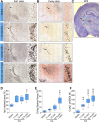
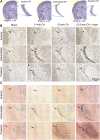
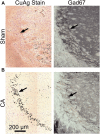
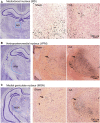
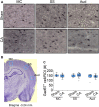
Children who survive cardiac arrest often develop debilitating sensorimotor and cognitive deficits. In animal models of cardiac arrest, delayed neuronal death in the hippocampal CA1 region has served as a fruitful paradigm for investigating mechanisms of injury and neuroprotection. Cardiac arrest in humans, however, is more prolonged than in most experimental models. Consequently, neurologic deficits in cardiac arrest survivors arise from injury not solely to CA1 but to multiple vulnerable brain structures. Here, we develop a rat model of prolonged pediatric asphyxial cardiac arrest and resuscitation, which better approximates arrest characteristics and injury severity in children. Using this model, we characterize features of microglial activation and neuronal degeneration in the thalamus 24 h after resuscitation from 11 and 12 min long cardiac arrest. In addition, we test the effect of mild hypothermia to 34°C for 8 h after 12.5 min of arrest. Microglial activation and neuronal degeneration are most prominent in the thalamic Reticular Nucleus (nRT). The severity of injury increases with increasing arrest duration, leading to frank loss of nRT neurons at longer arrest times. Hypothermia does not prevent nRT injury. Interestingly, injury occurs selectively in intermediate and posterior nRT segments while sparing the anterior segment. Since all nRT segments consist exclusively of GABA-ergic neurons, we asked if GABA-ergic neurons in general are more susceptible to hypoxic-ischemic injury. Surprisingly, cortical GABA-ergic neurons, like their counterparts in the anterior nRT segment, do not degenerate in this model. Hence, we propose that GABA-ergic identity alone is not sufficient to explain selective vulnerability of intermediate and posterior nRT neurons to hypoxic-ischemic injury after cardiac arrest and resuscitation. Our current findings align the animal model of pediatric cardiac arrest with human data and suggest novel mechanisms of selective vulnerability to hypoxic-ischemic injury among thalamic GABA-ergic neurons.
Keywords: GABA-ergic interneuron; cardiac arrest; hypoxia; ischemia; microglia; neuronal degeneration; reperfusion; thalamic reticular nucleus.
Copyright © 2021 Ton, Raffensperger and Shoykhet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Loss of parvalbumin immunoreactivity defines selectively vulnerable thalamic reticular nucleus neurons following cardiac arrest in the rat.Acta Neuropathol. 1995;89(3):262-9. doi: 10.1007/BF00309342. Acta Neuropathol. 1995. PMID: 7754747
-
Vulnerability of the thalamic somatosensory pathway after prolonged global hypoxic-ischemic injury.Neuroscience. 2002;115(3):917-29. doi: 10.1016/s0306-4522(02)00369-x. Neuroscience. 2002. PMID: 12435429
-
Thalamocortical dysfunction and thalamic injury after asphyxial cardiac arrest in developing rats.J Neurosci. 2012 Apr 4;32(14):4972-81. doi: 10.1523/JNEUROSCI.5597-11.2012. J Neurosci. 2012. PMID: 22492052 Free PMC article.
-
Resuscitative hypothermia.Crit Care Med. 1996 Feb;24(2 Suppl):S81-9. Crit Care Med. 1996. PMID: 8608709 Review.
-
Brain function after resuscitation from cardiac arrest.Curr Opin Crit Care. 2004 Jun;10(3):213-7. doi: 10.1097/01.ccx.0000127542.32890.fa. Curr Opin Crit Care. 2004. PMID: 15166839 Review.
Cited by
-
Post cardiac arrest physical exercise mitigates cell death in the septal and thalamic nuclei and ameliorates contextual fear conditioning deficits in rats.J Cereb Blood Flow Metab. 2023 Mar;43(3):446-459. doi: 10.1177/0271678X221137539. Epub 2022 Nov 11. J Cereb Blood Flow Metab. 2023. PMID: 36369732 Free PMC article.
-
Classification of Activated Microglia by Convolutional Neural Networks.IEEE Biomed Circuits Syst Conf. 2022 Oct;2022:198-202. doi: 10.1109/biocas54905.2022.9948635. Epub 2022 Nov 16. IEEE Biomed Circuits Syst Conf. 2022. PMID: 38544681 Free PMC article.
-
Thalamo-cortical neural mechanism of sodium salicylate-induced hyperacusis and anxiety-like behaviors.Commun Biol. 2024 Oct 18;7(1):1346. doi: 10.1038/s42003-024-07040-5. Commun Biol. 2024. PMID: 39420035 Free PMC article.
-
Lance-Adams Syndrome: Case series and literature review.Clin Neurophysiol Pract. 2023 Aug 18;8:187-193. doi: 10.1016/j.cnp.2023.08.002. eCollection 2023. Clin Neurophysiol Pract. 2023. PMID: 37822592 Free PMC article.
References
-
- Andy O. J., Jurko M. F. (1986). Seizure Control by Mesothalamic Reticular Stimulation. Clin. Electroencephalogr 17 (2), 52–60. - PubMed
-
- Au A. K., Chen Y., Du L., Smith C. M., Manole M. D., Baltagi S. A., et al. (2015). Ischemia-induced Autophagy Contributes to Neurodegeneration in Cerebellar Purkinje Cells in the Developing Rat Brain and in Primary Cortical Neurons In Vitro . Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1852 (9), 1902–1911. 10.1016/j.bbadis.2015.06.007 - DOI - PMC - PubMed
-
- Boyce-van der Wal L. W., Volker W. G., Vliet Vlieland T. P. M., van den Heuvel D. M. J., van Exel H. J., Goossens P. H. (2015). Cognitive Problems in Patients in a Cardiac Rehabilitation Program after an Out-Of-Hospital Cardiac Arrest. Resuscitation 93, 63–68. 10.1016/j.resuscitation.2015.05.029 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

