CXCR4 and CXCR7 Inhibition Ameliorates the Formation of Platelet-Neutrophil Complexes and Neutrophil Extracellular Traps through Adora2b Signaling
- PMID: 34948374
- PMCID: PMC8709064
- DOI: 10.3390/ijms222413576
CXCR4 and CXCR7 Inhibition Ameliorates the Formation of Platelet-Neutrophil Complexes and Neutrophil Extracellular Traps through Adora2b Signaling
Abstract
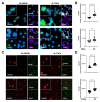
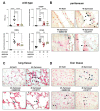
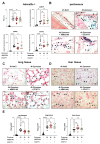
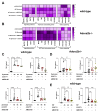
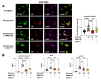
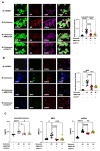
Peritonitis and peritonitis-associated sepsis are characterized by an increased formation of platelet-neutrophil complexes (PNCs), which contribute to an excessive migration of polymorphonuclear neutrophils (PMN) into the inflamed tissue. An important neutrophilic mechanism to capture and kill invading pathogens is the formation of neutrophil extracellular traps (NETs). Formation of PNCs and NETs are essential to eliminate pathogens, but also lead to aggravated tissue damage. The chemokine receptors CXCR4 and CXCR7 on platelets and PMNs have been shown to play a pivotal role in inflammation. Thereby, CXCR4 and CXCR7 were linked with functional adenosine A2B receptor (Adora2b) signaling. We evaluated the effects of selective CXCR4 and CXCR7 inhibition on PNCs and NETs in zymosan- and fecal-induced sepsis. We determined the formation of PNCs in the blood and, in addition, their infiltration into various organs in wild-type and Adora2b-/- mice by flow cytometry and histological methods. Further, we evaluated NET formation in both mouse lines and the impact of Adora2b signaling on it. We hypothesized that the protective effects of CXCR4 and CXCR7 antagonism on PNC and NET formation are linked with Adora2b signaling. We observed an elevated CXCR4 and CXCR7 expression in circulating platelets and PMNs during acute inflammation. Specific CXCR4 and CXCR7 inhibition reduced PNC formation in the blood, respectively, in the peritoneal, lung, and liver tissue in wild-type mice, while no protective anti-inflammatory effects were observed in Adora2b-/- animals. In vitro, CXCR4 and CXCR7 antagonism dampened PNC and NET formation with human platelets and PMNs, confirming our in vivo data. In conclusion, our study reveals new protective aspects of the pharmacological modulation of CXCR4 and CXCR7 on PNC and NET formation during acute inflammation.
Keywords: NETosis; PMNs; PNCs; SDF-1 receptors; acute inflammation; acute peritonitis; sepsis; thrombocytes.
Conflict of interest statement
The authors declare no conflict of interest. Chemocentryx provided the specific CXCR7 antagonist, CCX771, but was not involved in the study design or data evaluation.
Figures







Similar articles
-
Inhibition of CXCR4 and CXCR7 Is Protective in Acute Peritoneal Inflammation.Front Immunol. 2020 Mar 10;11:407. doi: 10.3389/fimmu.2020.00407. eCollection 2020. Front Immunol. 2020. PMID: 32210974 Free PMC article.
-
Inhibition of SDF-1 receptors CXCR4 and CXCR7 attenuates acute pulmonary inflammation via the adenosine A2B-receptor on blood cells.Cell Death Dis. 2017 May 25;8(5):e2832. doi: 10.1038/cddis.2016.482. Cell Death Dis. 2017. PMID: 28542132 Free PMC article.
-
CX3CR1 Depletion Promotes the Formation of Platelet-Neutrophil Complexes and Aggravates Acute Peritonitis.Shock. 2021 Aug 1;56(2):287-297. doi: 10.1097/SHK.0000000000001733. Shock. 2021. PMID: 33481549
-
Role of chemokine receptors CXCR4 and CXCR7 for platelet function.Biochem Soc Trans. 2015 Aug;43(4):720-6. doi: 10.1042/BST20150113. Epub 2015 Aug 3. Biochem Soc Trans. 2015. PMID: 26551719 Review.
-
Drug Design Targeting the CXCR4/CXCR7/CXCL12 Pathway.Curr Top Med Chem. 2016;16(13):1441-51. doi: 10.2174/1568026615666150915120218. Curr Top Med Chem. 2016. PMID: 26369824 Review.
Cited by
-
Platelets in the NETworks interweaving inflammation and thrombosis.Front Immunol. 2022 Aug 1;13:953129. doi: 10.3389/fimmu.2022.953129. eCollection 2022. Front Immunol. 2022. PMID: 35979369 Free PMC article. Review.
-
Atypical chemokine receptors in the immune system.Nat Rev Immunol. 2024 Oct;24(10):753-769. doi: 10.1038/s41577-024-01025-5. Epub 2024 May 7. Nat Rev Immunol. 2024. PMID: 38714818 Review.
-
Plerixafor for pathogen-agnostic treatment in murine thigh infection and zebrafish sepsis.Clin Transl Sci. 2024 Jul;17(7):e13876. doi: 10.1111/cts.13876. Clin Transl Sci. 2024. PMID: 38963161 Free PMC article.
-
Molecular mechanisms and therapeutic target of NETosis in diseases.MedComm (2020). 2022 Aug 19;3(3):e162. doi: 10.1002/mco2.162. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 36000086 Free PMC article. Review.
-
Pim Kinase Inhibition Disrupts CXCR4 Signalling in Megakaryocytes and Platelets by Reducing Receptor Availability at the Surface.Int J Mol Sci. 2024 Jul 11;25(14):7606. doi: 10.3390/ijms25147606. Int J Mol Sci. 2024. PMID: 39062849 Free PMC article.
References
-
- Coopersmith C.M., De Backer D., Deutschman C.S., Ferrer R., Lat I., Machado F.R., Martin G.S., Martin-Loeches I., Nunnally M.E., Antonelli M., et al. Surviving Sepsis Campaign: Research Priorities for Sepsis and Septic Shock. Crit. Care Med. 2018;46:1334–1356. doi: 10.1097/CCM.0000000000003225. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

