Advances in the Aetiology & Endoscopic Detection and Management of Early Gastric Cancer
- PMID: 34944861
- PMCID: PMC8699285
- DOI: 10.3390/cancers13246242
Advances in the Aetiology & Endoscopic Detection and Management of Early Gastric Cancer
Abstract
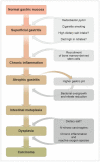
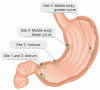
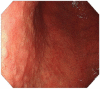
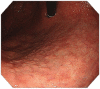
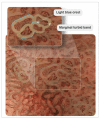
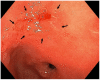
The mortality rates of gastric carcinoma remain high, despite the progress in research and development in disease mechanisms and treatment. Therefore, recognition of gastric precancerous lesions and early neoplasia is crucial. Two subtypes of sporadic gastric cancer have been recognized: cardia subtype and non-cardia (distal) subtype, the latter being more frequent and largely associated with infection of Helicobacter pylori, a class I carcinogen. Helicobacter pylori initiates the widely accepted Correa cascade, describing a stepwise progression through precursor lesions from chronic inflammation to gastric atrophy, gastric intestinal metaplasia and neoplasia. Our knowledge on He-licobacter pylori is still limited, and multiple questions in the context of its contribution to the pathogenesis of gastric neoplasia are yet to be answered. Awareness and recognition of gastric atrophy and intestinal metaplasia on high-definition white-light endoscopy, image-enhanced endoscopy and magnification endoscopy, in combination with histology from the biopsies taken accurately according to the protocol, are crucial to guiding the management. Standard indications for endoscopic resections (endoscopic mucosal resection and endoscopic submucosal dissection) of gastric dysplasia and intestinal type of gastric carcinoma have been recommended by multiple societies. Endoscopic evaluation and surveillance should be offered to individuals with an inherited predisposition to gastric carcinoma.
Keywords: Helicobacter pylori; chromoendoscopy; early gastric adenocarcinoma; endoscopic mucosal dissection; endoscopic mucosal resection; endoscopy; hereditary gastric adenocarcinoma; sporadic gastric adenocarcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
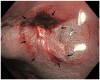
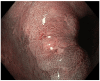
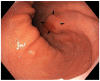
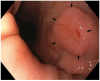

Similar articles
-
AGA Clinical Practice Update on Endoscopic Treatment of Barrett's Esophagus With Dysplasia and/or Early Cancer: Expert Review.Gastroenterology. 2020 Feb;158(3):760-769. doi: 10.1053/j.gastro.2019.09.051. Epub 2019 Nov 12. Gastroenterology. 2020. PMID: 31730766 Review.
-
Helicobacter pylori eradication may influence timing of endoscopic surveillance for gastric cancer in patients with gastric precancerous lesions: A retrospective study.Medicine (Baltimore). 2018 Jan;97(4):e9734. doi: 10.1097/MD.0000000000009734. Medicine (Baltimore). 2018. PMID: 29369216 Free PMC article.
-
Helicobacter pylori-induced inflammation masks the underlying presence of low-grade dysplasia on gastric lesions.World J Gastroenterol. 2020 Jul 14;26(26):3834-3850. doi: 10.3748/wjg.v26.i26.3834. World J Gastroenterol. 2020. PMID: 32774061 Free PMC article.
-
Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients.World J Gastroenterol. 2020 May 28;26(20):2618-2631. doi: 10.3748/wjg.v26.i20.2618. World J Gastroenterol. 2020. PMID: 32523315 Free PMC article.
-
Recent advances in the detection and management of early gastric cancer and its precursors.Frontline Gastroenterol. 2020 Jul 30;12(4):322-331. doi: 10.1136/flgastro-2018-101089. eCollection 2021. Frontline Gastroenterol. 2020. PMID: 34249318 Free PMC article. Review.
Cited by
-
Pyloric Gastric Adenoma: Endoscopic Detection, Removal, and Echoendosonographic Characterization.ACG Case Rep J. 2023 Dec 21;10(12):e01229. doi: 10.14309/crj.0000000000001229. eCollection 2023 Dec. ACG Case Rep J. 2023. PMID: 38130477 Free PMC article.
-
Gastric precancerous lesions:occurrence, development factors, and treatment.Front Oncol. 2023 Aug 30;13:1226652. doi: 10.3389/fonc.2023.1226652. eCollection 2023. Front Oncol. 2023. PMID: 37719006 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

