Small Endogenous Ligands Modulation of Nerve Growth Factor Bioactivity: A Structural Biology Overview
- PMID: 34943971
- PMCID: PMC8700322
- DOI: 10.3390/cells10123462
Small Endogenous Ligands Modulation of Nerve Growth Factor Bioactivity: A Structural Biology Overview
Abstract
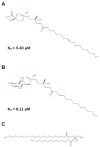
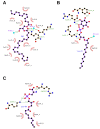
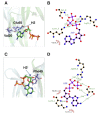
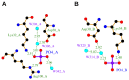
Experiments with cell cultures and animal models have provided solid support for the assumption that Nerve Growth Factor (NGF) plays a key role in the regulation of neuronal cell survival and death. Recently, endogenous ligands have been proposed as physiological modulators of NGF biological activity as part of this regulatory cascade. However, the structural and mechanistic determinants for NGF bioactivity remain to be elucidated. We recently unveiled, by an integrated structural biology approach, the ATP binding sites of NGF and investigated the effects on TrkA and p75NTR receptors binding. These results pinpoint ATP as a genuine endogenous modulator of NGF signaling, paving the way to the characterization of not-yet-identified chemical diverse endogenous biological active small molecules as novel modulators of NGF. The present review aims at providing an overview of the currently available 3D structures of NGF in complex with different small endogenous ligands, featuring the molecular footprints of the small molecules binding. This knowledge is essential for further understanding the functional role of small endogenous ligands in the modulation of neurotrophins signaling in physiological and pathological conditions and for better exploiting the therapeutic potentialities of NGF.
Keywords: ATP; bioactivity modulation; lysophospholipids; nerve growth factor; small endogenous ligands; zinc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Distinction between differentiation, cell cycle, and apoptosis signals in PC12 cells by the nerve growth factor mutant delta9/13, which is selective for the p75 neurotrophin receptor.J Neurosci Res. 2001 Jan 1;63(1):10-9. doi: 10.1002/1097-4547(20010101)63:1<10::AID-JNR2>3.0.CO;2-R. J Neurosci Res. 2001. PMID: 11169609
-
Comparison of nerve growth factor receptor binding models using heterodimeric muteins.J Neurosci Res. 2012 Dec;90(12):2259-71. doi: 10.1002/jnr.23116. Epub 2012 Aug 18. J Neurosci Res. 2012. PMID: 22903500 Free PMC article.
-
Nerve growth factor survival signaling in cultured hippocampal neurons is mediated through TrkA and requires the common neurotrophin receptor P75.Neuroscience. 2002;115(4):1089-108. doi: 10.1016/s0306-4522(02)00539-0. Neuroscience. 2002. PMID: 12453482
-
NGF and ProNGF: Regulation of neuronal and neoplastic responses through receptor signaling.Adv Biol Regul. 2015 May;58:16-27. doi: 10.1016/j.jbior.2014.11.003. Epub 2014 Nov 20. Adv Biol Regul. 2015. PMID: 25491371 Free PMC article. Review.
-
ATP binding to Nerve Growth Factor (NGF) and pro-Nerve Growth Factor (proNGF): an endogenous molecular switch modulating neurotrophins activity.Biochem Soc Trans. 2024 Jun 26;52(3):1293-1304. doi: 10.1042/BST20231089. Biochem Soc Trans. 2024. PMID: 38716884 Review.
Cited by
-
Distinct conformational changes occur within the intrinsically unstructured pro-domain of pro-Nerve Growth Factor in the presence of ATP and Mg2.Protein Sci. 2023 Feb;32(2):e4563. doi: 10.1002/pro.4563. Protein Sci. 2023. PMID: 36605018 Free PMC article.
-
Neurotrophic Activity and Its Modulation by Zinc Ion of a Dimeric Peptide Mimicking the Brain-Derived Neurotrophic Factor N-Terminal Region.ACS Chem Neurosci. 2022 Dec 7;13(23):3453-3463. doi: 10.1021/acschemneuro.2c00463. Epub 2022 Nov 8. ACS Chem Neurosci. 2022. PMID: 36346920 Free PMC article.
-
Heterogeneity-induced NGF-NGFR communication inefficiency promotes mitotic spindle disorganization in exhausted T cells through PREX1 suppression to impair the anti-tumor immunotherapy with PD-1 mAb in hepatocellular carcinoma.Cancer Med. 2024 Feb;13(3):e6736. doi: 10.1002/cam4.6736. Epub 2024 Jan 10. Cancer Med. 2024. PMID: 38204220 Free PMC article.
References
-
- Paoletti F., Merzel F., Cassetta A., Ogris I., Covaceuszach S., Grdadolnik J., Lamba D., Golič Grdadolnik S. Endogenous modulators of neurotrophin signaling: Landscape of the transient ATP-NGF interactions. Comput. Struct. Biotechnol. J. 2021;19:2938–2949. doi: 10.1016/j.csbj.2021.05.009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

