A Stochastic Spatiotemporal Model of Rat Ventricular Myocyte Calcium Dynamics Demonstrated Necessary Features for Calcium Wave Propagation
- PMID: 34940490
- PMCID: PMC8706945
- DOI: 10.3390/membranes11120989
A Stochastic Spatiotemporal Model of Rat Ventricular Myocyte Calcium Dynamics Demonstrated Necessary Features for Calcium Wave Propagation
Abstract
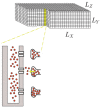
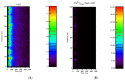
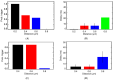
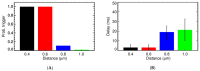
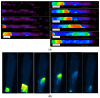
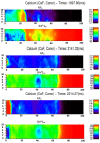
Calcium (Ca2+) plays a central role in the excitation and contraction of cardiac myocytes. Experiments have indicated that calcium release is stochastic and regulated locally suggesting the possibility of spatially heterogeneous calcium levels in the cells. This spatial heterogeneity might be important in mediating different signaling pathways. During more than 50 years of computational cell biology, the computational models have been advanced to incorporate more ionic currents, going from deterministic models to stochastic models. While periodic increases in cytoplasmic Ca2+ concentration drive cardiac contraction, aberrant Ca2+ release can underly cardiac arrhythmia. However, the study of the spatial role of calcium ions has been limited due to the computational expense of using a three-dimensional stochastic computational model. In this paper, we introduce a three-dimensional stochastic computational model for rat ventricular myocytes at the whole-cell level that incorporate detailed calcium dynamics, with (1) non-uniform release site placement, (2) non-uniform membrane ionic currents and membrane buffers, (3) stochastic calcium-leak dynamics and (4) non-junctional or rogue ryanodine receptors. The model simulates spark-induced spark activation and spark-induced Ca2+ wave initiation and propagation that occur under conditions of calcium overload at the closed-cell condition, but not when Ca2+ levels are normal. This is considered important since the presence of Ca2+ waves contribute to the activation of arrhythmogenic currents.
Keywords: arrhythmia; calcium waves; computational model; heart.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures






Similar articles
-
Modeling Calcium Cycling in the Heart: Progress, Pitfalls, and Challenges.Biomolecules. 2022 Nov 14;12(11):1686. doi: 10.3390/biom12111686. Biomolecules. 2022. PMID: 36421700 Free PMC article. Review.
-
Size Matters: Ryanodine Receptor Cluster Size Heterogeneity Potentiates Calcium Waves.Biophys J. 2019 Feb 5;116(3):530-539. doi: 10.1016/j.bpj.2018.12.017. Epub 2019 Jan 3. Biophys J. 2019. PMID: 30686487 Free PMC article.
-
Stochastic simulation of cardiac ventricular myocyte calcium dynamics and waves.Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:4677-80. doi: 10.1109/IEMBS.2011.6091158. Annu Int Conf IEEE Eng Med Biol Soc. 2011. PMID: 22255381 Free PMC article.
-
Ryanodine receptor sensitivity governs the stability and synchrony of local calcium release during cardiac excitation-contraction coupling.J Mol Cell Cardiol. 2016 Mar;92:82-92. doi: 10.1016/j.yjmcc.2016.01.024. Epub 2016 Jan 28. J Mol Cell Cardiol. 2016. PMID: 26827896 Free PMC article.
-
Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes.J Physiol. 2003 Jan 1;546(Pt 1):19-31. doi: 10.1113/jphysiol.2002.025239. J Physiol. 2003. PMID: 12509476 Free PMC article. Review.
Cited by
-
Pacing Dynamics Determines the Arrhythmogenic Mechanism of the CPVT2-Causing CASQ2G112+5X Mutation in a Guinea Pig Ventricular Myocyte Computational Model.Genes (Basel). 2022 Dec 22;14(1):23. doi: 10.3390/genes14010023. Genes (Basel). 2022. PMID: 36672764 Free PMC article.
-
Multi-Scale Computational Modeling of Spatial Calcium Handling From Nanodomain to Whole-Heart: Overview and Perspectives.Front Physiol. 2022 Mar 9;13:836622. doi: 10.3389/fphys.2022.836622. eCollection 2022. Front Physiol. 2022. PMID: 35370783 Free PMC article. Review.
-
Critical Requirements for the Initiation of a Cardiac Arrhythmia in Rat Ventricle: How Many Myocytes?Cells. 2022 Jun 9;11(12):1878. doi: 10.3390/cells11121878. Cells. 2022. PMID: 35741007 Free PMC article.
-
Modeling Calcium Cycling in the Heart: Progress, Pitfalls, and Challenges.Biomolecules. 2022 Nov 14;12(11):1686. doi: 10.3390/biom12111686. Biomolecules. 2022. PMID: 36421700 Free PMC article. Review.
-
Making time and space for calcium control of neuron activity.Curr Opin Neurobiol. 2023 Dec;83:102804. doi: 10.1016/j.conb.2023.102804. Epub 2023 Oct 31. Curr Opin Neurobiol. 2023. PMID: 37913687 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

