Structure-based identification of a new IAP-targeting compound that induces cancer cell death inducing NF-κB pathway
- PMID: 34938412
- PMCID: PMC8649670
- DOI: 10.1016/j.csbj.2021.11.034
Structure-based identification of a new IAP-targeting compound that induces cancer cell death inducing NF-κB pathway
Abstract
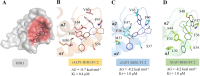
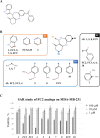
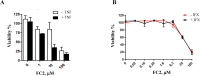
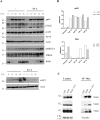
Inhibitors of apoptosis proteins (IAPs) are validated onco-targets, as their overexpression correlates with cancer onset, progression, diffusion and chemoresistance. IAPs regulate cell death survival pathways, inflammation, and immunity. Targeting IAPs, by impairing their protein-protein interaction surfaces, can affect events occurring at different stages of cancer development. To this purpose, we employed a rational virtual screening approach to identify compounds predicted to interfere with the assembly of pro-survival macromolecular complexes. One of the candidates, FC2, was shown to bind in vitro the BIR1 domains of both XIAP and cIAP2. Moreover, we demonstrated that FC2 can induce cancer cell death as a single agent and, more potently, in combination with the Smac-mimetic SM83 or with the cytokine TNF. FC2 determined a prolonged activation of the NF-κB pathway, accompanied to a stabilization of XIAP-TAB1 complex. This candidate molecule represents a valuable lead compound for the development of a new class of IAP-antagonists for cancer treatment.
Keywords: BIR, baculoviral IAP repeat; Baculoviral IAP repeat; Drug discovery; IAP, inhibitor of apoptosis proteins; IAP-antagonist; Inhibitor of apoptosis proteins; NF-kB; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; SM, Smac-mimetic; TAB, TAK1 binding protein; TAK1, (TGFβ)-activated kinase; TGFβ, transforming growth factor-β; TNF, tumor necrosis factor; TRAF, TNF receptor associated factor; Virtual screening.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
New Class of Benzodiazepinone Derivatives as Pro-Death Agents Targeting BIR Domains in Cancer Cells.Molecules. 2023 Jan 3;28(1):446. doi: 10.3390/molecules28010446. Molecules. 2023. PMID: 36615638 Free PMC article.
-
NF023 binding to XIAP-BIR1: searching drugs for regulation of the NF-κB pathway.Proteins. 2015 Apr;83(4):612-20. doi: 10.1002/prot.24766. Epub 2015 Feb 5. Proteins. 2015. PMID: 25619915
-
Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-κB activation, and is active in patient-derived xenograft models.Mol Cancer Ther. 2014 Apr;13(4):867-79. doi: 10.1158/1535-7163.MCT-13-0798. Epub 2014 Feb 21. Mol Cancer Ther. 2014. PMID: 24563541
-
IAP family of cell death and signaling regulators.Methods Enzymol. 2014;545:35-65. doi: 10.1016/B978-0-12-801430-1.00002-0. Methods Enzymol. 2014. PMID: 25065885 Review.
-
Inhibitor of apoptosis proteins (IAPs) as regulatory factors of hepatic apoptosis.Cell Signal. 2013 Oct;25(10):1970-80. doi: 10.1016/j.cellsig.2013.06.003. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770286 Review.
Cited by
-
New Class of Benzodiazepinone Derivatives as Pro-Death Agents Targeting BIR Domains in Cancer Cells.Molecules. 2023 Jan 3;28(1):446. doi: 10.3390/molecules28010446. Molecules. 2023. PMID: 36615638 Free PMC article.
-
ILP-2: A New Bane and Therapeutic Target for Human Cancers.Front Oncol. 2022 Jun 23;12:922596. doi: 10.3389/fonc.2022.922596. eCollection 2022. Front Oncol. 2022. PMID: 35814477 Free PMC article. Review.
-
Nanomaterials: A powerful tool for tumor immunotherapy.Front Immunol. 2022 Aug 22;13:979469. doi: 10.3389/fimmu.2022.979469. eCollection 2022. Front Immunol. 2022. PMID: 36072591 Free PMC article. Review.
-
Strontium Ranelate Inhibits Osteoclastogenesis through NF-κB-Pathway-Dependent Autophagy.Bioengineering (Basel). 2023 Mar 16;10(3):365. doi: 10.3390/bioengineering10030365. Bioengineering (Basel). 2023. PMID: 36978756 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
