SYT7 acts as an oncogene and a potential therapeutic target and was regulated by ΔNp63α in HNSCC
- PMID: 34930262
- PMCID: PMC8691088
- DOI: 10.1186/s12935-021-02394-w
SYT7 acts as an oncogene and a potential therapeutic target and was regulated by ΔNp63α in HNSCC
Abstract
Background: Head and neck squamous cell carcinoma (HNSCC) are one of the most common types of head and neck cancer, and it is urgent to find effective treatment for advanced patients. Exploring developing and progressing mechanisms of HNSCC could provide a theoretical basis to find new therapeutic targets.
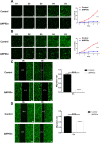
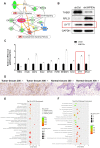
Methods: In our research, we performed a whole-gene expression profile microarray analysis to identify differential expression genes between squamous cell carcinoma cells and ΔNp63 alpha (ΔNp63α) knockdown cells. As a result, an important gene Synaptotagmin VII (SYT7) was screened out.
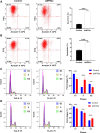
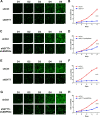
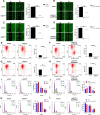
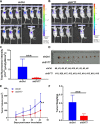
Results: SYT7 knockdown affected the proliferation, apoptosis and cell cycle of squamous cell carcinoma cells. The rescue experiment in vitro with ΔNp63α and SYT7 double knockdown resulted in partial reversion of ΔNp63α-induced phenotypes. This was also confirmed by experiments in vivo.
Conclusions: Taken together, we found that ΔNp63α could inhibit the occurrence and progression of HNSCC throughout downregulating the expression of SYT7. Therefore, SYT7/ΔNp63α axis could be a potential therapeutic target for clinical treatment of HNSCC.
Keywords: Head and neck squamous cell carcinoma; SYT7; TCGA, microarray; ΔNp63 alpha.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Dominant negative p63 isoform expression in head and neck squamous cell carcinoma.Laryngoscope. 2004 Dec;114(12):2063-72. doi: 10.1097/01.mlg.0000149437.35855.4b. Laryngoscope. 2004. PMID: 15564824
-
ΔNp63α promotes Bortezomib resistance via the CYGB-ROS axis in head and neck squamous cell carcinoma.Cell Death Dis. 2022 Apr 9;13(4):327. doi: 10.1038/s41419-022-04790-0. Cell Death Dis. 2022. PMID: 35397613 Free PMC article.
-
SATB2 augments ΔNp63α in head and neck squamous cell carcinoma.EMBO Rep. 2010 Oct;11(10):777-83. doi: 10.1038/embor.2010.125. Epub 2010 Sep 10. EMBO Rep. 2010. PMID: 20829881 Free PMC article.
-
Synaptotagmin7 Is Overexpressed In Colorectal Cancer And Regulates Colorectal Cancer Cell Proliferation.J Cancer. 2018 Jun 12;9(13):2349-2356. doi: 10.7150/jca.25098. eCollection 2018. J Cancer. 2018. PMID: 30026831 Free PMC article.
-
Calprotectin and the Initiation and Progression of Head and Neck Cancer.J Dent Res. 2018 Jun;97(6):674-682. doi: 10.1177/0022034518756330. Epub 2018 Feb 14. J Dent Res. 2018. PMID: 29443623 Free PMC article. Review.
Cited by
-
Intracellular Cholesterol Synthesis and Transport.Front Cell Dev Biol. 2022 Mar 21;10:819281. doi: 10.3389/fcell.2022.819281. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35386193 Free PMC article. Review.
-
Potential roles of synaptotagmin family members in cancers: Recent advances and prospects.Front Med (Lausanne). 2022 Aug 8;9:968081. doi: 10.3389/fmed.2022.968081. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36004367 Free PMC article. Review.
-
Circ_RNF13 Regulates the Stemness and Chemosensitivity of Colorectal Cancer by Transcriptional Regulation of DDX27 Mediated by TRIM24 Stabilization.Cancers (Basel). 2022 Dec 16;14(24):6218. doi: 10.3390/cancers14246218. Cancers (Basel). 2022. PMID: 36551703 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

