Antiviral Responses in Cancer: Boosting Antitumor Immunity Through Activation of Interferon Pathway in the Tumor Microenvironment
- PMID: 34925363
- PMCID: PMC8674309
- DOI: 10.3389/fimmu.2021.782852
Antiviral Responses in Cancer: Boosting Antitumor Immunity Through Activation of Interferon Pathway in the Tumor Microenvironment
Abstract
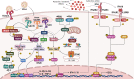
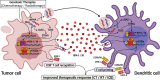
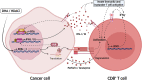
In recent years, it became apparent that cancers either associated with viral infections or aberrantly expressing endogenous retroviral elements (EREs) are more immunogenic, exhibiting an intense intra-tumor immune cell infiltration characterized by a robust cytolytic apparatus. On the other hand, epigenetic regulation of EREs is crucial to maintain steady-state conditions and cell homeostasis. In line with this, epigenetic disruptions within steady-state cells can lead to cancer development and trigger the release of EREs into the cytoplasmic compartment. As such, detection of viral molecules by intracellular innate immune sensors leads to the production of type I and type III interferons that act to induce an antiviral state, thus restraining viral replication. This knowledge has recently gained momentum due to the possibility of triggering intratumoral activation of interferon responses, which could be used as an adjuvant to elicit strong anti-tumor immune responses that ultimately lead to a cascade of cytokine production. Accordingly, several therapeutic approaches are currently being tested using this rationale to improve responses to cancer immunotherapies. In this review, we discuss the immune mechanisms operating in viral infections, show evidence that exogenous viruses and endogenous retroviruses in cancer may enhance tumor immunogenicity, dissect the epigenetic control of EREs, and point to interferon pathway activation in the tumor milieu as a promising molecular predictive marker and immunotherapy target. Finally, we briefly discuss current strategies to modulate these responses within tumor tissues, including the clinical use of innate immune receptor agonists and DNA demethylating agents.
Keywords: antitumor immunity; antiviral immune response; endogenous retroviral elements; epigenetic regulation; immunotherapy; interferons; oncolytic viruses.
Copyright © 2021 Vitiello, Ferreira, Cordeiro de Lima and Medina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Turning Cold into Hot: Firing up the Tumor Microenvironment.Trends Cancer. 2020 Jul;6(7):605-618. doi: 10.1016/j.trecan.2020.02.022. Epub 2020 Mar 21. Trends Cancer. 2020. PMID: 32610070 Review.
-
Intratumoral Immunotherapy: From Trial Design to Clinical Practice.Clin Cancer Res. 2021 Feb 1;27(3):665-679. doi: 10.1158/1078-0432.CCR-20-0473. Epub 2020 Sep 17. Clin Cancer Res. 2021. PMID: 32943460 Review.
-
Epigenetic Modifiers: Anti-Neoplastic Drugs With Immunomodulating Potential.Front Immunol. 2021 Mar 30;12:652160. doi: 10.3389/fimmu.2021.652160. eCollection 2021. Front Immunol. 2021. PMID: 33859645 Free PMC article. Review.
-
Cancer Epigenetics, Tumor Immunity, and Immunotherapy.Trends Cancer. 2020 Jul;6(7):580-592. doi: 10.1016/j.trecan.2020.02.003. Epub 2020 Mar 31. Trends Cancer. 2020. PMID: 32610068 Free PMC article. Review.
-
Current strategies for intratumoural immunotherapy - Beyond immune checkpoint inhibition.Eur J Cancer. 2021 Nov;157:493-510. doi: 10.1016/j.ejca.2021.08.004. Epub 2021 Sep 21. Eur J Cancer. 2021. PMID: 34561127 Review.
Cited by
-
Transcriptional and reverse transcriptional regulation of host genes by human endogenous retroviruses in cancers.Front Microbiol. 2022 Jul 19;13:946296. doi: 10.3389/fmicb.2022.946296. eCollection 2022. Front Microbiol. 2022. PMID: 35928153 Free PMC article. Review.
-
Comprehensive Analysis and Drug Modulation of Human Endogenous Retrovirus in Hepatocellular Carcinomas.Cancers (Basel). 2023 Jul 18;15(14):3664. doi: 10.3390/cancers15143664. Cancers (Basel). 2023. PMID: 37509325 Free PMC article.
-
Oncolytic Rodent Protoparvoviruses Evade a TLR- and RLR-Independent Antiviral Response in Transformed Cells.Pathogens. 2023 Apr 17;12(4):607. doi: 10.3390/pathogens12040607. Pathogens. 2023. PMID: 37111493 Free PMC article.
-
Neutralizing Antibodies Impair the Oncolytic Efficacy of Reovirus but Permit Effective Combination with T cell-Based Immunotherapies.Cancer Immunol Res. 2024 Mar 4;12(3):334-349. doi: 10.1158/2326-6066.CIR-23-0480. Cancer Immunol Res. 2024. PMID: 38194598 Free PMC article.
-
Involvement of APOBEC3A/B Deletion in Mouse Mammary Tumor Virus (MMTV)-like Positive Human Breast Cancer.Diagnostics (Basel). 2023 Mar 22;13(6):1196. doi: 10.3390/diagnostics13061196. Diagnostics (Basel). 2023. PMID: 36980505 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

