Committed Human CD23-Negative Light-Zone Germinal Center B Cells Delineate Transcriptional Program Supporting Plasma Cell Differentiation
- PMID: 34925321
- PMCID: PMC8674954
- DOI: 10.3389/fimmu.2021.744573
Committed Human CD23-Negative Light-Zone Germinal Center B Cells Delineate Transcriptional Program Supporting Plasma Cell Differentiation
Abstract
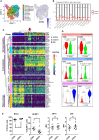
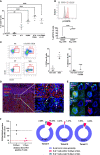
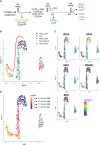
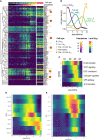
B cell affinity maturation occurs in the germinal center (GC). Light-zone (LZ) GC B cells (BGC-cells) interact with follicular dendritic cells (FDCs) and compete for the limited, sequential help from T follicular helper cells needed to escape from apoptosis and complete their differentiation. The highest-affinity LZ BGC-cells enter the cell cycle and differentiate into PCs, following a dramatic epigenetic reorganization that induces transcriptome changes in general and the expression of the PRDM1 gene in particular. Human PC precursors are characterized by the loss of IL-4/STAT6 signaling and the absence of CD23 expression. Here, we studied the fate of human LZ BGC-cells as a function of their CD23 expression. We first showed that CD23 expression was restricted to the GC LZ, where it was primarily expressed by FDCs; less than 10% of tonsil LZ BGC-cells were positive. Sorted LZ BGC-cells left in culture and stimulated upregulated CD23 expression but were unable to differentiate into PCs - in contrast to cells that did not upregulate CD23 expression. An in-depth analysis (including single-cell gene expression) showed that stimulated CD23-negative LZ BGC-cells differentiated into plasmablasts and time course of gene expression changes delineates the transcriptional program that sustains PC differentiation. In particular, we identified a B cell proliferation signature supported by a transient MYC gene expression. Overall, the CD23 marker might be of value in answering questions about the differentiation of normal BGC-cells and allowed us to propose an instructive LZ BGC-cells maturation and fate model.
Keywords: B cell differentiation; CD23+ B cells; GC Light-Zone B cells; germinal center (GC); germinal center (GC) B cells; plasmablasts/plasma cells.
Copyright © 2021 Santamaria, Desmots, Leonard, Caron, Haas, Delaloy, Chatonnet, Rossille, Pignarre, Monvoisin, Seffals, Lamaison, Cogné, Tarte and Fest.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Expression of the Plasma Cell Transcriptional Regulator Blimp-1 by Dark Zone Germinal Center B Cells During Periods of Proliferation.Front Immunol. 2019 Jan 9;9:3106. doi: 10.3389/fimmu.2018.03106. eCollection 2018. Front Immunol. 2019. PMID: 30687317 Free PMC article.
-
Germinal center B-cells.Autoimmunity. 2012 Aug;45(5):333-47. doi: 10.3109/08916934.2012.665524. Epub 2012 Apr 2. Autoimmunity. 2012. PMID: 22390182 Review.
-
Plasma cells, plasmablasts, and AID+/CD30+ B lymphoblasts inside and outside germinal centres: details of the basal light zone and the outer zone in human palatine tonsils.Histochem Cell Biol. 2020 Jul;154(1):55-75. doi: 10.1007/s00418-020-01861-1. Epub 2020 Mar 14. Histochem Cell Biol. 2020. PMID: 32172287 Free PMC article.
-
T Follicular Helper Cell-Germinal Center B Cell Interaction Strength Regulates Entry into Plasma Cell or Recycling Germinal Center Cell Fate.Immunity. 2018 Apr 17;48(4):702-715.e4. doi: 10.1016/j.immuni.2018.03.027. Immunity. 2018. PMID: 29669250
-
Heterogeneity of germinal center B cells: New insights from single-cell studies.Eur J Immunol. 2021 Nov;51(11):2555-2567. doi: 10.1002/eji.202149235. Epub 2021 Aug 8. Eur J Immunol. 2021. PMID: 34324199 Review.
Cited by
-
From neglect to spotlight: the underappreciated role of B cells in cutaneous inflammatory diseases.Front Immunol. 2024 Feb 15;15:1328785. doi: 10.3389/fimmu.2024.1328785. eCollection 2024. Front Immunol. 2024. PMID: 38426103 Free PMC article. Review.
-
Integrative single-cell chromatin and transcriptome analysis of human plasma cell differentiation.Blood. 2024 Aug 1;144(5):496-509. doi: 10.1182/blood.2023023237. Blood. 2024. PMID: 38643512 Free PMC article.
-
High-plex protein and whole transcriptome co-mapping at cellular resolution with spatial CITE-seq.Nat Biotechnol. 2023 Oct;41(10):1405-1409. doi: 10.1038/s41587-023-01676-0. Epub 2023 Feb 23. Nat Biotechnol. 2023. PMID: 36823353 Free PMC article.
-
BHLHE41, a transcriptional repressor involved in physiological processes and tumor development.Cell Oncol (Dordr). 2024 Sep 10. doi: 10.1007/s13402-024-00973-3. Online ahead of print. Cell Oncol (Dordr). 2024. PMID: 39254779 Review.
-
Epstein-Barr virus perpetuates B cell germinal center dynamics and generation of autoimmune-associated phenotypes in vitro.Front Immunol. 2022 Sep 28;13:1001145. doi: 10.3389/fimmu.2022.1001145. eCollection 2022. Front Immunol. 2022. PMID: 36248899 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

