Cell fusion as a link between the SARS-CoV-2 spike protein, COVID-19 complications, and vaccine side effects
- PMID: 34917266
- PMCID: PMC8664391
- DOI: 10.18632/oncotarget.28088
Cell fusion as a link between the SARS-CoV-2 spike protein, COVID-19 complications, and vaccine side effects
Abstract
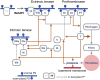
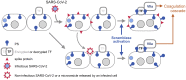
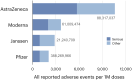
A distinctive feature of the SARS-CoV-2 spike protein is its ability to efficiently fuse cells, thus producing syncytia found in COVID-19 patients. This commentary proposes how this ability enables spike to cause COVID-19 complications as well as side effects of COVID-19 vaccines, and suggests how these effects can be prevented.
Keywords: cancer; cell fusion; neuropathy; thrombosis; vaccines.
Copyright: © 2021 Lazebnik.
Conflict of interest statement
CONFLICTS OF INTEREST Author have no conflicts of interest to declare.
Figures
Similar articles
-
Changes within the P681 residue of spike dictate cell fusion and syncytia formation of Delta and Omicron variants of SARS-CoV-2 with no effects on neutralization or infectivity.Heliyon. 2023 Jun;9(6):e16750. doi: 10.1016/j.heliyon.2023.e16750. Epub 2023 Jun 3. Heliyon. 2023. PMID: 37292300 Free PMC article.
-
Impairment of SARS-CoV-2 spike glycoprotein maturation and fusion activity by nitazoxanide: an effect independent of spike variants emergence.Cell Mol Life Sci. 2022 Apr 7;79(5):227. doi: 10.1007/s00018-022-04246-w. Cell Mol Life Sci. 2022. PMID: 35391601 Free PMC article.
-
The British variant of the new coronavirus-19 (Sars-Cov-2) should not create a vaccine problem.J Biol Regul Homeost Agents. 2021 Jan-Feb;35(1):1-4. doi: 10.23812/21-3-E. J Biol Regul Homeost Agents. 2021. PMID: 33377359
-
Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection.Viruses. 2023 Apr 25;15(5):1045. doi: 10.3390/v15051045. Viruses. 2023. PMID: 37243131 Free PMC article. Review.
-
SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines.Vaccines (Basel). 2021 Jan 11;9(1):36. doi: 10.3390/vaccines9010036. Vaccines (Basel). 2021. PMID: 33440640 Free PMC article. Review.
Cited by
-
COVID-19, Vaccines, and Thrombotic Events: A Narrative Review.J Clin Med. 2022 Feb 11;11(4):948. doi: 10.3390/jcm11040948. J Clin Med. 2022. PMID: 35207220 Free PMC article. Review.
-
Identification of oral therapeutics using an AI platform against the virus responsible for COVID-19, SARS-CoV-2.Front Pharmacol. 2023 Dec 22;14:1297924. doi: 10.3389/fphar.2023.1297924. eCollection 2023. Front Pharmacol. 2023. PMID: 38186640 Free PMC article.
-
COVID-19-The Shift of Homeostasis into Oncopathology or Chronic Fibrosis in Terms of Female Reproductive System Involvement.Int J Mol Sci. 2023 May 11;24(10):8579. doi: 10.3390/ijms24108579. Int J Mol Sci. 2023. PMID: 37239926 Free PMC article. Review.
-
Sporadic Kaposi Sarcoma Following a COVID-19 Vaccine: Mere Coincidence or Something More?Cureus. 2024 Feb 9;16(2):e53925. doi: 10.7759/cureus.53925. eCollection 2024 Feb. Cureus. 2024. PMID: 38465101 Free PMC article.
References
-
- Bussani R, Schneider E, Zentilin L, Collesi C, Ali H, Braga L, Volpe MC, Colliva A, Zanconati F, Berlot G, Silvestri F, Zacchigna S, Giacca M. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020; 61:103104. 10.1016/j.ebiom.2020.103104. - DOI - PMC - PubMed
-
- Braga L, Ali H, Secco I, Chiavacci E, Neves G, Goldhill D, Penn R, Jimenez-Guardeño JM, Ortega-Prieto AM, Bussani R, Cannatà A, Rizzari G, Collesi C, et al.. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature. 2021; 594:88–93. 10.1038/s41586-021-03491-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

