Role of GluA4 in the acoustic and tactile startle responses
- PMID: 34915397
- PMCID: PMC8776314
- DOI: 10.1016/j.heares.2021.108410
Role of GluA4 in the acoustic and tactile startle responses
Abstract
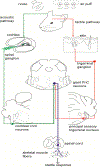
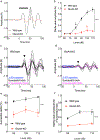
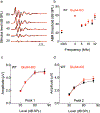
The primary startle response (SR) is an innate reaction evoked by sudden and intense acoustic, tactile or visual stimuli. In rodents and humans the SR involves reflexive contractions of the face, neck and limb muscles. The acoustic startle response (ASR) pathway consists of auditory nerve fibers (AN), cochlear root neurons (CRNs) and giant neurons of the caudal pontine reticular nucleus (PnC), which synapse on cranial and spinal motor neurons. The tactile startle response (TSR) is transmitted by primary sensory neurons to the principal sensory (Pr5) and spinal (Sp5) trigeminal nuclei. The ventral part of Pr5 projects directly to the PnC neurons. The SR requires rapid transmission of sensory information to initiate a fast motor response. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) are necessary to transmit auditory information to the PnC neurons and elicit the SR. AMPARs containing the glutamate AMPAR subunit 4 (GluA4) have fast kinetics, which makes them ideal candidates to transmit the SR signal. This study examined the role of GluA4 within the primary SR pathway by using GluA4 knockout (GluA4-KO) mice. Deletion of GluA4 considerably decreased the amplitude and probability of successful ASR and TSR, indicating that the presence of this subunit is critical at a common station within the startle pathway. We conclude that deletion of GluA4 affects the transmission of sensory signals from acoustic and tactile pathways to the motor component of the startle reflex. Therefore, GluA4 is required for the full response and for reliable elicitation of the startle response.
Keywords: AMPAR; Caudal pontine reticular nucleus (PnC); GluA4; Principal sensory trigeminal nucleus (Pr5); Startle reflex.
Copyright © 2021. Published by Elsevier B.V.
Figures
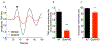

Similar articles
-
Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit?J Neurosci. 1994 Mar;14(3 Pt 1):1176-94. doi: 10.1523/JNEUROSCI.14-03-01176.1994. J Neurosci. 1994. PMID: 8120618 Free PMC article.
-
Cellular mechanisms of the trigeminally evoked startle response.Eur J Neurosci. 2003 Apr;17(7):1438-44. doi: 10.1046/j.1460-9568.2003.02565.x. Eur J Neurosci. 2003. PMID: 12713646
-
A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis.J Neurosci. 1996 Jun 1;16(11):3775-89. doi: 10.1523/JNEUROSCI.16-11-03775.1996. J Neurosci. 1996. PMID: 8642420 Free PMC article.
-
Tactile, acoustic and vestibular systems sum to elicit the startle reflex.Neurosci Biobehav Rev. 2002 Jan;26(1):1-11. doi: 10.1016/s0149-7634(01)00057-4. Neurosci Biobehav Rev. 2002. PMID: 11835980 Review.
-
The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation.Behav Brain Res. 1997 Dec;89(1-2):35-49. doi: 10.1016/s0166-4328(97)02296-1. Behav Brain Res. 1997. PMID: 9475613 Review.
Cited by
-
Molecular and functional profiling of cell diversity and identity in the lateral superior olive, an auditory brainstem center with ascending and descending projections.Front Cell Neurosci. 2024 May 23;18:1354520. doi: 10.3389/fncel.2024.1354520. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38846638 Free PMC article.
-
Long-Term Capsaicin Administration Ameliorates the Dysfunction and Astrogliosis of the Brain in Aged Mice with Missing Maxillary Molars.Nutrients. 2023 May 25;15(11):2471. doi: 10.3390/nu15112471. Nutrients. 2023. PMID: 37299434 Free PMC article.
-
Co-coding of head and whisker movements by both VPM and POm thalamic neurons.Nat Commun. 2024 Jul 13;15(1):5883. doi: 10.1038/s41467-024-50039-z. Nat Commun. 2024. PMID: 39003286 Free PMC article.
-
Reduced Serum Levels of Soluble Interleukin-15 Receptor α in Schizophrenia and Its Relationship to the Excited Phenotype.Front Psychiatry. 2022 Mar 9;13:842003. doi: 10.3389/fpsyt.2022.842003. eCollection 2022. Front Psychiatry. 2022. PMID: 35356722 Free PMC article.
-
GluA3 subunits are required for appropriate assembly of AMPAR GluA2 and GluA4 subunits on cochlear afferent synapses and for presynaptic ribbon modiolar-pillar morphology.Elife. 2023 Jan 17;12:e80950. doi: 10.7554/eLife.80950. Elife. 2023. PMID: 36648432 Free PMC article.
References
-
- Berg WK, Balaban MT 1999. Startle elicitation: stimulus parameters, recording techniques, and quantification. In: Startle modification: Implications for neuroscience, cognitive science, and clinical science. Eds. Dawson ME, Schell AM, Bohmelt AH Cambridge University Press. pp. 21–50.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

