Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia
- PMID: 34914826
- PMCID: PMC8952188
- DOI: 10.1182/blood.2021013290
Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia
Abstract
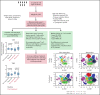
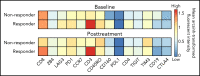
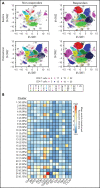
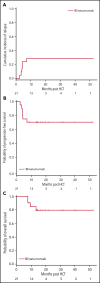
Patients with B-lineage acute lymphoblastic leukemia (ALL) are at high-risk for relapse after allogeneic hematopoietic cell transplantation (HCT). We conducted a single-center phase 2 study evaluating the feasibility of 4 cycles of blinatumomab administered every 3 months during the first year after HCT in an effort to mitigate relapse in high-risk ALL patients. Twenty-one of 23 enrolled patients received at least 1 cycle of blinatumomab and were included in the analysis. The median time from HCT to the first cycle of blinatumomab was 78 days (range, 44 to 105). Twelve patients (57%) completed all 4 treatment cycles. Neutropenia was the only grade 4 adverse event (19%). Rates of cytokine release (5% G1) and neurotoxicity (5% G2) were minimal. The cumulative incidence of acute graft-versus-host disease (GVHD) grades 2 to 4 and 3 to 4 were 33% and 5%, respectively; 2 cases of mild (10%) and 1 case of moderate (5%) chronic GVHD were noted. With a median follow-up of 14.3 months, the 1-year overall survival (OS), progression-free survival (PFS), and nonrelapse mortality (NRM) rates were 85%, 71%, and 0%, respectively. In a matched analysis with a contemporary cohort of 57 patients, we found no significant difference between groups regarding blinatumomab's efficacy. Correlative studies of baseline and posttreatment samples identified patients with specific T-cell profiles as "responders" or "nonresponders" to therapy. Responders had higher proportions of effector memory CD8 T-cell subsets. Nonresponders were T-cell deficient and expressed more inhibitory checkpoint molecules, including T-cell immunoglobulin and mucin domain 3 (TIM3). We found that blinatumomab postallogeneic HCT is feasible, and its benefit is dependent on the immune milieu at time of treatment. This paper is posted on ClinicalTrials.gov, study ID: NCT02807883.
© 2022 by The American Society of Hematology.
Figures
Similar articles
-
Blinatumomab Therapy Is Associated with Favorable Outcomes after Allogeneic Hematopoietic Cell Transplantation in Pediatric Patients with B Cell Acute Lymphoblastic Leukemia.Transplant Cell Ther. 2024 Feb;30(2):217-227. doi: 10.1016/j.jtct.2023.10.024. Epub 2023 Nov 4. Transplant Cell Ther. 2024. PMID: 37931800
-
Pretransplant Blinatumomab Improves Outcomes in B Cell Acute Lymphoblastic Leukemia Patients Who Undergo Allogeneic Hematopoietic Cell Transplantation.Transplant Cell Ther. 2024 May;30(5):520.e1-520.e12. doi: 10.1016/j.jtct.2024.03.004. Epub 2024 Mar 8. Transplant Cell Ther. 2024. PMID: 38462215
-
Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children With High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial.JAMA. 2021 Mar 2;325(9):843-854. doi: 10.1001/jama.2021.0987. JAMA. 2021. PMID: 33651091 Free PMC article. Clinical Trial.
-
Comparison of reduced-intensity and myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia and acute lymphoblastic leukemia: a meta-analysis.Stem Cells Dev. 2014 Nov 1;23(21):2535-52. doi: 10.1089/scd.2014.0123. Epub 2014 Sep 17. Stem Cells Dev. 2014. PMID: 25072307 Free PMC article. Review.
-
SOHO State of the Art Updates and Next Questions | Optimal Timing of Blinatumomab for the Treatment of B-Acute Lymphoblastic Leukemia.Clin Lymphoma Myeloma Leuk. 2023 Mar;23(3):159-167. doi: 10.1016/j.clml.2022.12.011. Epub 2022 Dec 25. Clin Lymphoma Myeloma Leuk. 2023. PMID: 36642665 Review.
Cited by
-
Outcomes of allogeneic hematopoietic cell transplantation in adults with fusions associated with Ph-like ALL.Blood Adv. 2022 Sep 13;6(17):4936-4948. doi: 10.1182/bloodadvances.2022007597. Blood Adv. 2022. PMID: 35816633 Free PMC article.
-
Immunotherapy for Pediatric Acute Lymphoblastic Leukemia: Recent Advances and Future Perspectives.Front Immunol. 2022 Jun 13;13:921894. doi: 10.3389/fimmu.2022.921894. eCollection 2022. Front Immunol. 2022. PMID: 35769486 Free PMC article. Review.
-
CD8+ CD28- regulatory T cells after induction therapy predict progression-free survival in myeloma patients: results from the GMMG-HD6 multicenter phase III study.Leukemia. 2024 Jul;38(7):1621-1625. doi: 10.1038/s41375-024-02290-y. Epub 2024 Jun 3. Leukemia. 2024. PMID: 38830959 Free PMC article. Clinical Trial. No abstract available.
-
Safety and efficacy of post-haematopoietic cell transplantation maintenance therapy with blinatumomab for relapsed/refractory CD19-positive B-cell acute lymphoblastic leukaemia: protocol for a phase I-II, multicentre, non-blinded, non-controlled trial (JPLSG SCT-ALL-BLIN21).BMJ Open. 2023 Apr 17;13(4):e070051. doi: 10.1136/bmjopen-2022-070051. BMJ Open. 2023. PMID: 37068890 Free PMC article.
-
Survival outcomes in patients with relapsed/refractory or MRD-positive B-cell acute lymphoblastic leukemia treated with blinatumomab.Ther Adv Hematol. 2023 Oct 9;14:20406207231201454. doi: 10.1177/20406207231201454. eCollection 2023. Ther Adv Hematol. 2023. PMID: 37822571 Free PMC article. Review.
References
-
- Goldstone AH, Richards SM, Lazarus HM, et al. . In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. - PubMed
-
- Fielding AK, Rowe JM, Richards SM, et al. . Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113(19):4489-4496. - PMC - PubMed
-
- Gökbuget N, Kneba M, Raff T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia . Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9): 1868-1876. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

