SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021
- PMID: 34914670
- PMCID: PMC8675659
- DOI: 10.15585/mmwr.mm7050e1
SARS-CoV-2 B.1.1.529 (Omicron) Variant - United States, December 1-8, 2021
Abstract
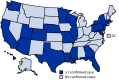
A new variant of SARS-CoV-2 (the virus that causes COVID-19), B.1.1.529 (Omicron) (1), was first reported to the World Health Organization (WHO) by South Africa on November 24, 2021. Omicron has numerous mutations with potential to increase transmissibility, confer resistance to therapeutics, or partially escape infection- or vaccine-induced immunity (2). On November 26, WHO designated B.1.1.529 as a variant of concern (3), as did the U.S. SARS-CoV-2 Interagency Group (SIG)* on November 30. On December 1, the first case of COVID-19 attributed to the Omicron variant was reported in the United States. As of December 8, a total of 22 states had identified at least one Omicron variant case, including some that indicate community transmission. Among 43 cases with initial follow-up, one hospitalization and no deaths were reported. This report summarizes U.S. surveillance for SARS-CoV-2 variants, characteristics of the initial persons investigated with COVID-19 attributed to the Omicron variant and public health measures implemented to slow the spread of Omicron in the United States. Implementation of concurrent prevention strategies, including vaccination, masking, increasing ventilation, testing, quarantine, and isolation, are recommended to slow transmission of SARS-CoV-2, including variants such as Omicron, and to protect against severe illness and death from COVID-19.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures
Similar articles
-
Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics.Eur Rev Med Pharmacol Sci. 2021 Dec;25(24):8012-8018. doi: 10.26355/eurrev_202112_27652. Eur Rev Med Pharmacol Sci. 2021. PMID: 34982465
-
Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods - United States, December 2020-January 2022.MMWR Morb Mortal Wkly Rep. 2022 Jan 28;71(4):146-152. doi: 10.15585/mmwr.mm7104e4. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35085225 Free PMC article.
-
Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants - United States, June 2021-January 2022.MMWR Morb Mortal Wkly Rep. 2022 Feb 11;71(6):206-211. doi: 10.15585/mmwr.mm7106a4. MMWR Morb Mortal Wkly Rep. 2022. PMID: 35143464 Free PMC article.
-
Omicron (B.1.1.529) - variant of concern - molecular profile and epidemiology: a mini review.Eur Rev Med Pharmacol Sci. 2021 Dec;25(24):8019-8022. doi: 10.26355/eurrev_202112_27653. Eur Rev Med Pharmacol Sci. 2021. PMID: 34982466 Review.
-
SARS-CoV-2 Omicron Mutation Is Faster than the Chase: Multiple Mutations on Spike/ACE2 Interaction Residues.Immune Netw. 2021 Dec 23;21(6):e38. doi: 10.4110/in.2021.21.e38. eCollection 2021 Dec. Immune Netw. 2021. PMID: 35036025 Free PMC article. Review.
Cited by
-
An expanded RT-PCR melting temperature coding assay to rapidly identify all known SARS-CoV-2 variants and sub-variants of concern.Sci Rep. 2023 Dec 11;13(1):21927. doi: 10.1038/s41598-023-48647-8. Sci Rep. 2023. PMID: 38081834 Free PMC article.
-
Nirmatrelvir/Ritonavir and Molnupiravir in the Treatment of Mild/Moderate COVID-19: Results of a Real-Life Study.Vaccines (Basel). 2022 Oct 17;10(10):1731. doi: 10.3390/vaccines10101731. Vaccines (Basel). 2022. PMID: 36298596 Free PMC article.
-
Computational investigation of natural compounds as potential main protease (Mpro) inhibitors for SARS-CoV-2 virus.Comput Biol Med. 2022 Dec;151(Pt A):106318. doi: 10.1016/j.compbiomed.2022.106318. Epub 2022 Nov 18. Comput Biol Med. 2022. PMID: 36423529 Free PMC article.
-
Application of animal models to compare and contrast the virulence of current and future potential SARS-CoV-2 variants.Biosaf Health. 2022 Jun;4(3):154-160. doi: 10.1016/j.bsheal.2022.05.001. Epub 2022 May 5. Biosaf Health. 2022. PMID: 35528630 Free PMC article. Review.
-
Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic.Vaccines (Basel). 2022 May 11;10(5):755. doi: 10.3390/vaccines10050755. Vaccines (Basel). 2022. PMID: 35632512 Free PMC article. Review.
References
-
- CDC. Science brief: Omicron (B.1.1.529) variant. Atlanta, GA: US Department of Health and Human Services, CDC; 2021. Accessed December 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scienti...
-
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Geneva, Switzerland: World Health Organization; 2021. Accessed December 3, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....
-
- University of Texas at Austin. User’s guide to variant detection calculators. Austin, TX: University of Texas at Austin; 2021. https://covid-19.tacc.utexas.edu/media/filer_public/d9/d9/d9d99a16-704e-...
-
- ThermoFisher Scientific. TaqPath COVID-19 Multiplex Diagnostic Solution. Waltham, MA: ThermoFisher Scientific; 2021. Accessed December 3, 2021. https://www.thermofisher.com/us/en/home/clinical/clinical-genomics/patho...
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous