Specificity protein 1/microRNA-92b forms a feedback loop promoting the migration and invasion of head and neck squamous cell carcinoma
- PMID: 34905435
- PMCID: PMC8810166
- DOI: 10.1080/21655979.2021.2008698
Specificity protein 1/microRNA-92b forms a feedback loop promoting the migration and invasion of head and neck squamous cell carcinoma
Abstract
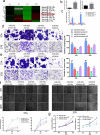
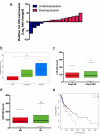
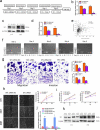
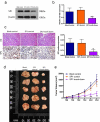
In this study we report a novel specificity protein 1 (SP1)/microRNA-92b (miR-92b) feedback loop regulating the migration and invasion of head and neck squamous cell carcinoma (HNSCC). Microarray and real-time Polymerase Chain Reaction (PCR) were used to detect gene expression in HNSCC tissues and cell lines. Transwell migration, invasion, wound healing and cell counting kit - 8 (CCK-8) cell assays were used to compare cell migration, invasion and proliferation abilities. Chromatin Immunoprecipitation (ChIP) assays were used to detect SP1 binding to the miR-92b promoter. Western blot was used to detect protein levels. An in vivo tumorigenesis experiment was used to evaluate the effect of SP1 knockdown on tumor growth and protein levels were evaluated by immunohistochemistry. We found that the miR-92b expression level was elevated in HNSCC primary focus tissue compared with adjacent normal tissue, and a higher level of miR-92b was related to a higher clinical stage and worse prognosis of HNSCC patients. MiR-92b and SP1 mutually promoted each expression and cooperatively facilitated the migration, invasion and proliferation of HNSCC cells. A decreased level of SP1/miR-92b resulted in a restraint of in vivo tumor growth. In conclusion, our results suggest that the SP1/miR-92b feedback loop generally promotes HNSCC invasion and metastasis, thus presenting a possible therapeutic target in the treatment of HNSCC patients.
Keywords: Head and neck squamous cell carcinoma; feedback loop; microRNA-92b; migration and invasion; specificity protein 1.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
[Role of specificity protein 1 in transcription regulation of microRNA-92b in head and neck squamous cell carcinoma].Zhonghua Kou Qiang Yi Xue Za Zhi. 2017 Sep 9;52(9):563-568. doi: 10.3760/cma.j.issn.1002-0098.2017.09.011. Zhonghua Kou Qiang Yi Xue Za Zhi. 2017. PMID: 29972923 Chinese.
-
A Study on the Correlations of the miR-31 Expression with the Pathogenesis and Prognosis of Head and Neck Squamous Cell Carcinoma.Cancer Biother Radiopharm. 2019 Apr;34(3):189-195. doi: 10.1089/cbr.2018.2621. Epub 2019 Jan 10. Cancer Biother Radiopharm. 2019. PMID: 30628842
-
Long noncoding RNA MEG3 decreases the growth of head and neck squamous cell carcinoma by regulating the expression of miR-421 and E-cadherin.Cancer Med. 2020 Jun;9(11):3954-3963. doi: 10.1002/cam4.3002. Epub 2020 Apr 11. Cancer Med. 2020. PMID: 32277605 Free PMC article.
-
Comprehensive review regarding the association of E2Fs with the prognosis and immune infiltrates in human head and neck squamous cell carcinoma.Asian J Surg. 2024 May;47(5):2106-2121. doi: 10.1016/j.asjsur.2024.01.130. Epub 2024 Feb 5. Asian J Surg. 2024. PMID: 38320907 Review.
-
The Prognostic Role of miR-31 in Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-Analysis with Trial Sequential Analysis.Int J Environ Res Public Health. 2022 Apr 27;19(9):5334. doi: 10.3390/ijerph19095334. Int J Environ Res Public Health. 2022. PMID: 35564727 Free PMC article. Review.
Cited by
-
Specificity protein 1/3 regulate T-cell acute lymphoblastic leukemia cell proliferation and apoptosis through β-catenin by acting as targets of miR-495-3p.Ann Hematol. 2024 Aug;103(8):2945-2960. doi: 10.1007/s00277-024-05764-2. Epub 2024 Jun 3. Ann Hematol. 2024. PMID: 38829410
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Wilkie MD, Chudek DA, Flynn CD, et al. Outcomes and prognosticators in regionally recurrent cutaneous squamous cell carcinoma of the head and neck. Eur J Surg Oncol. 2020;46(11):2035–2041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
