Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics
- PMID: 34901546
- PMCID: PMC8636666
- DOI: 10.1016/j.bioactmat.2021.08.029
Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics
Abstract
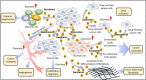
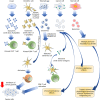
Cancer is a deadly disease that is globally and consistently one of the leading causes of mortality every year. Despite the availability of chemotherapy, radiotherapy, immunotherapy, and surgery, a cure for cancer has not been attained. Recently, exosomes have gained significant attention due to the therapeutic potential of their various components including proteins, lipids, nucleic acids, miRNAs, and lncRNAs. Exosomes constitute a set of tiny extracellular vesicles with an approximate diameter of 30-100 nm. They are released from different cells and are present in biofluids including blood, cerebrospinal fluid (CSF), and urine. They perform crucial multifaceted functions in the malignant progression of cancer via autocrine, paracrine, and endocrine communications. The ability of exosomes to carry different cargoes including drug and molecular information to recipient cells make them a novel tool for cancer therapeutics. In this review, we discuss the major components of exosomes and their role in cancer progression. We also review important literature about the potential role of exosomes as vaccines and delivery carriers in the context of cancer therapeutics.
Keywords: Cancer; Exosomal delivery system; Exosomal vaccine; Exosome; Extracellular vesicles; Therapeutics.
© 2021 The Authors.
Conflict of interest statement
None.
Figures



Similar articles
-
Exosomes: Diagnostic and Therapeutic Implications in Cancer.Pharmaceutics. 2023 May 11;15(5):1465. doi: 10.3390/pharmaceutics15051465. Pharmaceutics. 2023. PMID: 37242707 Free PMC article. Review.
-
Tumor-derived extracellular vesicles: reliable tools for Cancer diagnosis and clinical applications.Cell Commun Signal. 2019 Jul 10;17(1):73. doi: 10.1186/s12964-019-0390-y. Cell Commun Signal. 2019. PMID: 31291956 Free PMC article. Review.
-
Exosomics in oral cancer diagnosis, prognosis, and therapeutics - An emergent and imperative non-invasive natural nanoparticle-based approach.Crit Rev Oncol Hematol. 2022 Oct;178:103799. doi: 10.1016/j.critrevonc.2022.103799. Epub 2022 Aug 27. Crit Rev Oncol Hematol. 2022. PMID: 36031170 Review.
-
Possibility of Exosome-Based Therapeutics and Challenges in Production of Exosomes Eligible for Therapeutic Application.Biol Pharm Bull. 2018;41(6):835-842. doi: 10.1248/bpb.b18-00133. Biol Pharm Bull. 2018. PMID: 29863072 Review.
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
Cited by
-
Co-delivery of Liposomal Ketoconazole and Bevacizumab for Synergistical Inhibition of Angiogenesis Against Endometrial Cancer.Mol Biotechnol. 2024 Sep 4. doi: 10.1007/s12033-024-01227-1. Online ahead of print. Mol Biotechnol. 2024. PMID: 39230827
-
Exosomal miRNA-146a-5p Derived from Senescent Hepatocellular Carcinoma Cells Promotes Aging and Inhibits Aerobic Glycolysis in Liver Cells via Targeting IRF7.J Cancer. 2024 Jun 17;15(14):4448-4466. doi: 10.7150/jca.96500. eCollection 2024. J Cancer. 2024. PMID: 39006088 Free PMC article.
-
Modulatory effects of cancer stem cell-derived extracellular vesicles on the tumor immune microenvironment.Front Immunol. 2024 Jun 19;15:1362120. doi: 10.3389/fimmu.2024.1362120. eCollection 2024. Front Immunol. 2024. PMID: 38962016 Free PMC article. Review.
-
Exosome nanovesicles as potential biomarkers and immune checkpoint signaling modulators in lung cancer microenvironment: recent advances and emerging concepts.J Exp Clin Cancer Res. 2023 Aug 29;42(1):221. doi: 10.1186/s13046-023-02753-7. J Exp Clin Cancer Res. 2023. PMID: 37641132 Free PMC article. Review.
-
The Roles of Exosomes as Future Therapeutic Agents and Diagnostic Tools for Glioma.Front Oncol. 2021 Oct 13;11:733529. doi: 10.3389/fonc.2021.733529. eCollection 2021. Front Oncol. 2021. PMID: 34722277 Free PMC article. Review.
References
-
- Xu C., Thakur A., Li Z., Yang T., Zhao C., Li Y., Lee Y., Wu C.-M.L. Determination of glioma cells' malignancy and their response to TMZ via detecting exosomal BIGH3 by a TiO2-CTFE-AuNIs plasmonic biosensor. Chem. Eng. J. 2021;415:128948. doi: 10.1016/j.cej.2021.128948. - DOI
Publication types
LinkOut - more resources
Full Text Sources

