An Overview of in vivo Functions of Chondroitin Sulfate and Dermatan Sulfate Revealed by Their Deficient Mice
- PMID: 34901009
- PMCID: PMC8652114
- DOI: 10.3389/fcell.2021.764781
An Overview of in vivo Functions of Chondroitin Sulfate and Dermatan Sulfate Revealed by Their Deficient Mice
Abstract
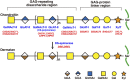
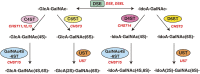
Chondroitin sulfate (CS), dermatan sulfate (DS) and heparan sulfate (HS) are covalently attached to specific core proteins to form proteoglycans in their biosynthetic pathways. They are constructed through the stepwise addition of respective monosaccharides by various glycosyltransferases and maturated by epimerases as well as sulfotransferases. Structural diversities of CS/DS and HS are essential for their various biological activities including cell signaling, cell proliferation, tissue morphogenesis, and interactions with a variety of growth factors as well as cytokines. Studies using mice deficient in enzymes responsible for the biosynthesis of the CS/DS and HS chains of proteoglycans have demonstrated their essential functions. Chondroitin synthase 1-deficient mice are viable, but exhibit chondrodysplasia, progression of the bifurcation of digits, delayed endochondral ossification, and reduced bone density. DS-epimerase 1-deficient mice show thicker collagen fibrils in the dermis and hypodermis, and spina bifida. These observations suggest that CS/DS are essential for skeletal development as well as the assembly of collagen fibrils in the skin, and that their respective knockout mice can be utilized as models for human genetic disorders with mutations in chondroitin synthase 1 and DS-epimerase 1. This review provides a comprehensive overview of mice deficient in CS/DS biosyntheses.
Keywords: chondroitin sulfate; dermatan sulfate; epimerase; glycosyltransferase; knockout mouse; proteoglycan; sulfotransferase; transporter.
Copyright © 2021 Mizumoto and Yamada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Histories of Dermatan Sulfate Epimerase and Dermatan 4-O-Sulfotransferase from Discovery of Their Enzymes and Genes to Musculocontractural Ehlers-Danlos Syndrome.Genes (Basel). 2023 Feb 16;14(2):509. doi: 10.3390/genes14020509. Genes (Basel). 2023. PMID: 36833436 Free PMC article. Review.
-
The Specific Role of Dermatan Sulfate as an Instructive Glycosaminoglycan in Tissue Development.Int J Mol Sci. 2022 Jul 5;23(13):7485. doi: 10.3390/ijms23137485. Int J Mol Sci. 2022. PMID: 35806490 Free PMC article. Review.
-
Biosynthesis and function of chondroitin sulfate.Biochim Biophys Acta. 2013 Oct;1830(10):4719-33. doi: 10.1016/j.bbagen.2013.06.006. Epub 2013 Jun 14. Biochim Biophys Acta. 2013. PMID: 23774590 Review.
-
Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders.Pharmaceuticals (Basel). 2017 Mar 27;10(2):34. doi: 10.3390/ph10020034. Pharmaceuticals (Basel). 2017. PMID: 28346368 Free PMC article. Review.
-
Chondroitin / dermatan sulfate modification enzymes in zebrafish development.PLoS One. 2015 Mar 20;10(3):e0121957. doi: 10.1371/journal.pone.0121957. eCollection 2015. PLoS One. 2015. PMID: 25793894 Free PMC article.
Cited by
-
Characterization of Hyaluronidase 4 Involved in the Catabolism of Chondroitin Sulfate.Molecules. 2022 Sep 18;27(18):6103. doi: 10.3390/molecules27186103. Molecules. 2022. PMID: 36144836 Free PMC article.
-
A Method for Bridging Population-Specific Genotypes to Detect Gene Modules Associated with Alzheimer's Disease.Cells. 2022 Jul 16;11(14):2219. doi: 10.3390/cells11142219. Cells. 2022. PMID: 35883662 Free PMC article.
-
Control of tissue homeostasis by the extracellular matrix: Synthetic heparan sulfate as a promising therapeutic for periodontal health and bone regeneration.Periodontol 2000. 2024 Feb;94(1):510-531. doi: 10.1111/prd.12515. Epub 2023 Aug 24. Periodontol 2000. 2024. PMID: 37614159 Free PMC article. Review.
-
Mice lacking nucleotide sugar transporter SLC35A3 exhibit lethal chondrodysplasia with vertebral anomalies and impaired glycosaminoglycan biosynthesis.PLoS One. 2023 Apr 13;18(4):e0284292. doi: 10.1371/journal.pone.0284292. eCollection 2023. PLoS One. 2023. PMID: 37053259 Free PMC article.
-
Genetics of glycosylation in mammalian development and disease.Nat Rev Genet. 2024 Oct;25(10):715-729. doi: 10.1038/s41576-024-00725-x. Epub 2024 May 9. Nat Rev Genet. 2024. PMID: 38724711 Review.
References
-
- Bartolini B., Thelin M. A., Rauch U., Feinstein R., Oldberg A., Malmström A., et al. (2012). Mouse Development Is Not Obviously Affected by the Absence of Dermatan Sulfate Epimerase 2 in Spite of a Modified Brain Dermatan Sulfate Composition. Glycobiology 22, 1007–1016. 10.1093/glycob/cws065 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

