Recombinant Human Bone Morphogenic Protein-2 Immobilized Fabrication of Magnesium Functionalized Injectable Hydrogels for Controlled-Delivery and Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells in Femoral Head Necrosis Repair
- PMID: 34900987
- PMCID: PMC8656218
- DOI: 10.3389/fcell.2021.723789
Recombinant Human Bone Morphogenic Protein-2 Immobilized Fabrication of Magnesium Functionalized Injectable Hydrogels for Controlled-Delivery and Osteogenic Differentiation of Rat Bone Marrow-Derived Mesenchymal Stem Cells in Femoral Head Necrosis Repair
Abstract
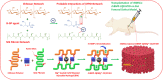
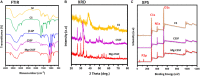
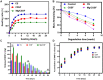
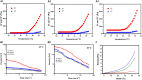
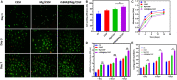
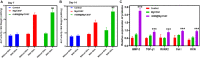
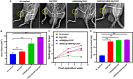
Femoral head necrosis (FHN) is a clinically progressive disease that leads to overwhelming complications without an effective therapeutic approach. In recent decades, transplantation of mesenchymal stem cells (MSCs) has played a promising role in the treatment of FHN in the initial stage; however, the success rate is still low because of unsuitable cell carriers and abridged osteogenic differentiation of the transplanted MSCs. Biopolymeric-derived hydrogels have been extensively applied as effective cell carriers and drug vesicles; they provide the most promising contributions in the fields of tissue engineering and regenerative medicine. However, the clinical potential of hydrogels may be limited because of inappropriate gelation, swelling, mechanical characteristics, toxicity in the cross-linking process, and self-healing ability. Naturally, gelated commercial hydrogels are not suitable for cell injection and infiltration because of their static network structure. In this study, we designed a novel thermogelling injectable hydrogel using natural silk fibroin-blended chitosan (CS) incorporated with magnesium (Mg) substitutes to improve physical cross-linking, stability, and cell osteogenic compatibility. The presented observations demonstrate that the developed injectable hydrogels can facilitate the controlled delivery of immobilized recombinant human bone morphogenic protein-2 (rhBMP-2) and rat bone marrow-derived MSCs (rBMSCs) with greater cell encapsulation efficiency, compatibility, and osteogenic differentiation. In addition, outcomes of in vivo animal studies established promising osteoinductive, bone mineral density, and bone formation rate after implantation of the injectable hydrogel scaffolds. Therefore, the developed hydrogels have great potential for clinical applications of FHN therapy.
Keywords: cell encapsulation; femoral head necrosis; injectable hydrogel; magnesium; stem cells.
Copyright © 2021 Lu, Guo, Li, Sun and Xiong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Synergic adhesive chemistry-based fabrication of BMP-2 immobilized silk fibroin hydrogel functionalized with hybrid nanomaterial to augment osteogenic differentiation of rBMSCs for bone defect repair.Int J Biol Macromol. 2021 Dec 1;192:407-416. doi: 10.1016/j.ijbiomac.2021.09.036. Epub 2021 Sep 28. Int J Biol Macromol. 2021. PMID: 34597700
-
Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering.Acta Biomater. 2019 Sep 1;95:348-356. doi: 10.1016/j.actbio.2019.02.046. Epub 2019 Mar 1. Acta Biomater. 2019. PMID: 30831326
-
An injectable hydroxypropyl-β-cyclodextrin cross-linked gelatin-based hydrogel loaded bone mesenchymal stem cell for osteogenic and in vivo bone regeneration of femoral head necrosis.Nanomedicine. 2022 Apr;41:102521. doi: 10.1016/j.nano.2022.102521. Epub 2022 Jan 13. Nanomedicine. 2022. PMID: 35032630
-
Advanced Hydrogels as Exosome Delivery Systems for Osteogenic Differentiation of MSCs: Application in Bone Regeneration.Int J Mol Sci. 2021 Jun 8;22(12):6203. doi: 10.3390/ijms22126203. Int J Mol Sci. 2021. PMID: 34201385 Free PMC article. Review.
-
Biopolymeric In Situ Hydrogels for Tissue Engineering and Bioimaging Applications.Tissue Eng Regen Med. 2018 Sep 14;15(5):575-590. doi: 10.1007/s13770-018-0159-1. eCollection 2018 Oct. Tissue Eng Regen Med. 2018. PMID: 30603580 Free PMC article. Review.
Cited by
-
Harnessing the Stem Cell Niche in Regenerative Medicine: Innovative Avenue to Combat Neurodegenerative Diseases.Int J Mol Sci. 2024 Jan 12;25(2):993. doi: 10.3390/ijms25020993. Int J Mol Sci. 2024. PMID: 38256066 Free PMC article. Review.
-
Hydrogel Use in Osteonecrosis of the Femoral Head.Gels. 2024 Aug 22;10(8):544. doi: 10.3390/gels10080544. Gels. 2024. PMID: 39195073 Free PMC article. Review.
-
Biodegradable Magnesium Biomaterials-Road to the Clinic.Bioengineering (Basel). 2022 Mar 5;9(3):107. doi: 10.3390/bioengineering9030107. Bioengineering (Basel). 2022. PMID: 35324796 Free PMC article. Review.
-
Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering.Polymers (Basel). 2022 May 27;14(11):2184. doi: 10.3390/polym14112184. Polymers (Basel). 2022. PMID: 35683857 Free PMC article. Review.
-
A Review of the Role of Tendon Stem Cells in Tendon-Bone Regeneration.Med Sci Monit. 2023 Sep 16;29:e940805. doi: 10.12659/MSM.940805. Med Sci Monit. 2023. PMID: 37715366 Free PMC article. Review.
References
-
- Bedair T. M., Lee C. K., Kim D. S., Baek S. W., Bedair H. M., Joshi H. P., et al. (2020). Magnesium hydroxide-incorporated PLGA composite attenuates inflammation and promotes BMP2-induced bone formation in spinal fusion. J. Tissue Eng. 11:2041731420967591. 10.1177/2041731420967591 - DOI - PMC - PubMed
-
- Chen C. Y., Du W., Rao S. S., Tan Y. J., Hu X. K., Luo M. J., et al. (2020). Extracellular vesicles from human urine-derived stem cells inhibit glucocorticoid-induced osteonecrosis of the femoral head by transporting and releasing pro-angiogenic DMBT1 and anti-apoptotic TIMP1. Acta Biomater. 111 208–220. 10.1016/j.actbio.2020.05.020 - DOI - PubMed
LinkOut - more resources
Full Text Sources

