Arming Immune Cell Therapeutics with Polymeric Prodrugs
- PMID: 34889072
- PMCID: PMC9847575
- DOI: 10.1002/adhm.202101944
Arming Immune Cell Therapeutics with Polymeric Prodrugs
Abstract
Engineered immune cells are an exciting therapeutic modality, which survey and attack tumors. Backpacking strategies exploit cell targeting capabilities for delivery of drugs to combat tumors and their immune-suppressive environments. Here, a new platform for arming cell therapeutics through dual receptor and polymeric prodrug engineering is developed. Macrophage and T cell therapeutics are engineered to express a bioorthogonal single chain variable fragment receptor. The receptor binds a fluorescein ligand that directs cell loading with ligand-tagged polymeric prodrugs, termed "drugamers." The fluorescein ligand facilitates stable binding of drugamer to engineered macrophages over 10 days with 80% surface retention. Drugamers also incorporate prodrug monomers of the phosphoinositide-3-kinase inhibitor, PI-103. The extended release of PI-103 from the drugamer sustains antiproliferative activity against a glioblastoma cell line compared to the parent drug. The versatility and modularity of this cell arming system is demonstrated by loading T cells with a second fluorescein-drugamer. This drugamer incorporates a small molecule estrogen analog, CMP8, which stabilizes a degron-tagged transgene to provide temporal regulation of protein activity in engineered T cells. These results demonstrate that this bioorthogonal receptor and drugamer system can be used to arm multiple immune cell classes with both antitumor and transgene-activating small molecule prodrugs.
Keywords: cell backpacking; cell-mediated targeting; macrophages; polymeric prodrugs; solid tumors.
© 2021 Wiley-VCH GmbH.
Figures




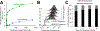
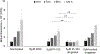
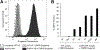
Similar articles
-
Macrophage-targeted drugamers with enzyme-cleavable linkers deliver high intracellular drug dosing and sustained drug pharmacokinetics against alveolar pulmonary infections.J Control Release. 2018 Oct 10;287:1-11. doi: 10.1016/j.jconrel.2018.08.014. Epub 2018 Aug 9. J Control Release. 2018. PMID: 30099019 Free PMC article.
-
Multifunctional Polymeric Prodrug with Simultaneous Conjugating Camptothecin and Doxorubicin for pH/Reduction Dual-Responsive Drug Delivery.ACS Appl Mater Interfaces. 2019 Mar 6;11(9):8740-8748. doi: 10.1021/acsami.8b16363. Epub 2019 Feb 20. ACS Appl Mater Interfaces. 2019. PMID: 30693750
-
A polymeric prodrug of 5-fluorouracil-1-acetic acid using a multi-hydroxyl polyethylene glycol derivative as the drug carrier.PLoS One. 2014 Nov 12;9(11):e112888. doi: 10.1371/journal.pone.0112888. eCollection 2014. PLoS One. 2014. PMID: 25389968 Free PMC article.
-
Release from polymeric prodrugs: linkages and their degradation.J Pharm Sci. 2004 Aug;93(8):1962-79. doi: 10.1002/jps.20096. J Pharm Sci. 2004. PMID: 15236447 Review.
-
Prodrug strategy for cancer cell-specific targeting: A recent overview.Eur J Med Chem. 2017 Oct 20;139:542-563. doi: 10.1016/j.ejmech.2017.08.010. Epub 2017 Aug 4. Eur J Med Chem. 2017. PMID: 28837920 Review.
Cited by
-
Tumor-associated macrophages in nanomaterial-based anti-tumor therapy: as target spots or delivery platforms.Front Bioeng Biotechnol. 2023 Aug 16;11:1248421. doi: 10.3389/fbioe.2023.1248421. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37654704 Free PMC article. Review.
-
Cell-mediated nanoparticle delivery systems: towards precision nanomedicine.Drug Deliv Transl Res. 2024 Nov;14(11):3032-3054. doi: 10.1007/s13346-024-01591-0. Epub 2024 Apr 13. Drug Deliv Transl Res. 2024. PMID: 38615157 Free PMC article. Review.
References
-
- Fuso Nerini I, Morosi L, Zucchetti M, Ballerini A, Giavazzi R, D’Incalci M, Intratumor heterogeneity and its impact on drug distribution and sensitivity, Clin Pharmacol Ther, 96 (2014) 224–238. - PubMed
-
- Minchinton AI, Tannock IF, Drug penetration in solid tumours, Nat Rev Cancer, 6 (2006) 583–592. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

