Amelioration of human peritoneal mesothelial cell co-culture-evoked malignant potential of ovarian cancer cells by acacetin involves LPA release-activated RAGE-PI3K/AKT signaling
- PMID: 34886812
- PMCID: PMC8903696
- DOI: 10.1186/s11658-021-00296-3
Amelioration of human peritoneal mesothelial cell co-culture-evoked malignant potential of ovarian cancer cells by acacetin involves LPA release-activated RAGE-PI3K/AKT signaling
Abstract
Background: Ovarian cancer is a devastating gynecological malignancy and frequently presents as an advanced carcinoma with disseminated peritoneum metastasis. Acacetin exerts anti-cancerous effects in several carcinomas. Here, we sought to investigate acacetin function in ovarian cancer malignancy triggered by peritoneal mesothelial cells.
Methods: Peritoneal mesothelial cells were treated with acacetin, and then the conditioned medium was collected to treat ovarian cancer cells. Then, cell proliferation was analyzed by MTT assay. Transwell analysis was conducted to evaluate cell invasion. Protein expression was determined by western blotting. ELISA and qRT-PCR were applied to analyze inflammatory cytokine levels. The underlying mechanism was also explored.
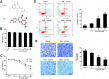
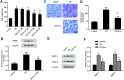
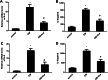
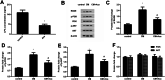
Results: Acacetin suppressed cell proliferation and invasion, but enhanced cell apoptosis. Furthermore, mesothelial cell-evoked malignant characteristics were inhibited when mesothelial cells were pre-treated with acacetin via restraining cell proliferation and invasion, concomitant with decreases in proliferation-related PCNA, MMP-2 and MMP-9 levels. Simultaneously, acacetin reduced mesothelial cell-induced transcripts and production of pro-inflammatory cytokine IL-6 and IL-8 in ovarian cancer cells. Mechanically, acacetin decreased lysophosphatidic acid (LPA) release from mesothelial cells, and subsequent activation of receptor for advanced glycation end-products (RAGE)-PI3K/AKT signaling in ovarian cancer cells. Notably, exogenous LPA restored the above pathway, and offset the efficacy of acacetin against mesothelial cell-evoked malignancy in ovarian cancer cells, including cell proliferation, invasion and inflammatory cytokine production.
Conclusions: Acacetin may not only engender direct inhibition of ovarian cancer cell malignancy, but also antagonize mesothelial cell-evoked malignancy by blocking LPA release-activated RAGE-PI3K/AKT signaling. Thus, these findings provide supporting evidence for a promising therapeutic agent against ovarian cancer.
Keywords: Acacetin; Cell invasion; Cell proliferation; Ovarian cancer; PI3K/AKT; Peritoneal mesothelial cells.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells.Cancer Res. 2006 Mar 15;66(6):3006-14. doi: 10.1158/0008-5472.CAN-05-1292. Cancer Res. 2006. PMID: 16540649
-
Acacetin inhibits invasion, migration and TGF-β1-induced EMT of gastric cancer cells through the PI3K/Akt/Snail pathway.BMC Complement Med Ther. 2022 Jan 9;22(1):10. doi: 10.1186/s12906-021-03494-w. BMC Complement Med Ther. 2022. PMID: 35000605 Free PMC article.
-
Anticancer effects of α-mangostin in OVACAR-3 human ovarian carcinoma cells are mediated via involvement of reactive oxygen species, mitochondrial -mediated apoptosis, suppression of cell migration and invasion and m-TOR/PI3K/AKT signaling pathway.J BUON. 2020 Sep-Oct;25(5):2293-2300. J BUON. 2020. PMID: 33277848
-
The multifaced role and therapeutic regulation of autophagy in ovarian cancer.Clin Transl Oncol. 2023 May;25(5):1207-1217. doi: 10.1007/s12094-022-03045-w. Epub 2022 Dec 19. Clin Transl Oncol. 2023. PMID: 36534371 Review.
-
Cancer-mesothelial and cancer-macrophage interactions in the ovarian cancer microenvironment.Am J Physiol Cell Physiol. 2023 Sep 1;325(3):C721-C730. doi: 10.1152/ajpcell.00461.2022. Epub 2023 Aug 7. Am J Physiol Cell Physiol. 2023. PMID: 37545408 Free PMC article. Review.
Cited by
-
The role of cancer-associated mesothelial cells in the progression and therapy of ovarian cancer.Front Immunol. 2022 Oct 4;13:1013506. doi: 10.3389/fimmu.2022.1013506. eCollection 2022. Front Immunol. 2022. PMID: 36268019 Free PMC article. Review.
-
The microRNA Let-7 and its exosomal form: Epigenetic regulators of gynecological cancers.Cell Biol Toxicol. 2024 Jun 5;40(1):42. doi: 10.1007/s10565-024-09884-3. Cell Biol Toxicol. 2024. PMID: 38836981 Free PMC article. Review.
-
Glycation and a Spark of ALEs (Advanced Lipoxidation End Products) - Igniting RAGE/Diaphanous-1 and Cardiometabolic Disease.Front Cardiovasc Med. 2022 Jun 24;9:937071. doi: 10.3389/fcvm.2022.937071. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35811725 Free PMC article. Review.
-
Targeting interleukin-6 as a treatment approach for peritoneal carcinomatosis.J Transl Med. 2024 Apr 30;22(1):402. doi: 10.1186/s12967-024-05205-8. J Transl Med. 2024. PMID: 38689325 Free PMC article. Review.
-
Micropattern Silk Fibroin Film Facilitates Tendon Repair In Vivo and Promotes Tenogenic Differentiation of Tendon Stem/Progenitor Cells through the α2β1/FAK/PI3K/AKT Signaling Pathway In Vitro.Stem Cells Int. 2023 Jan 13;2023:2915826. doi: 10.1155/2023/2915826. eCollection 2023. Stem Cells Int. 2023. PMID: 36684388 Free PMC article.
References
-
- Saika K, Sobue T. Cancer statistics in the world. Gan To Kagaku Ryoho. 2013;40:2475–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

