Imidazole Analogs of Vascular-Disrupting Combretastatin A-4 with Pleiotropic Efficacy against Resistant Colorectal Cancer Models
- PMID: 34884888
- PMCID: PMC8658273
- DOI: 10.3390/ijms222313082
Imidazole Analogs of Vascular-Disrupting Combretastatin A-4 with Pleiotropic Efficacy against Resistant Colorectal Cancer Models
Abstract
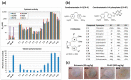
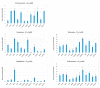
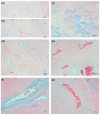
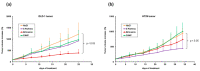
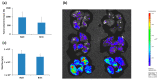
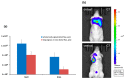
Specific targeting of the tumoral vasculature by vascular-disrupting agents (VDA), of which combretastatin A-4 (CA-4) is a main representative, has been considered a new therapeutic strategy against multidrug-resistant tumors. In addition, CA-4 and analogs are tubulin-targeting agents and can exert direct antitumor effects by different mechanisms. Herein, we analyzed a series of synthetic CA-4 analogs featuring N-methylimidazole-bridged Z-alkenes with different halo- or amino-substituted aryl rings in vitro and in vivo, focusing on models of colorectal cancer. Combined in vitro/in vivo structure-activity relationship studies using cell lines and xenograft tumors susceptible to VDA-induced vascular damage demonstrated a clear association of cytotoxic and vascular-disrupting activity with the ability to inhibit tubulin polymerization, which was determined by specific substitution constellations. The most active compounds were tested in an extended panel of colorectal cancer (CRC) cell lines and showed activity in CA-4-resistant and chemotherapy-resistant cell lines. The bromo derivative brimamin was then compared with the known fosbretabulin (CA-4P) by activity tests on DLD-1- (multidrug-resistant) and HT29- (CA-4-resistant) derived xenograft tumors. Treatment did not induce pronounced vascular-disrupting effects in these tumors. Histological analyses revealed distinct tumor substructures and vessel compositions of DLD-1/HT29 tumors, which clearly differed from the tumor models susceptible to VDA treatment. Even so, brimamin effectively retarded the growth of DLD-1 tumors, overcoming their resistance to standard treatment, and it inhibited the outgrowth of disseminated HT29 tumor cells in an experimental metastasis model. In conclusion, combretastatin analogous N-methylimidazoles proved capable of inducing vascular-disrupting effects, comparable to those of CA-4P. In addition, they showed antitumor activities in models of drug-resistant colorectal cancer, independent of vascular-disrupting effects.
Keywords: chemotherapy resistance; colorectal cancer; combretastatin A-4; imidazoles; microtubule destabilization; vascular-disrupting agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
4-(3-Halo/amino-4,5-dimethoxyphenyl)-5-aryloxazoles and -N-methylimidazoles that are cytotoxic against combretastatin A resistant tumor cells and vascular disrupting in a cisplatin resistant germ cell tumor model.J Med Chem. 2010 Sep 23;53(18):6595-602. doi: 10.1021/jm100345r. J Med Chem. 2010. PMID: 20731355
-
Combretastatin A-4 derived 5-(1-methyl-4-phenyl-imidazol-5-yl)indoles with superior cytotoxic and anti-vascular effects on chemoresistant cancer cells and tumors.Eur J Med Chem. 2016 Aug 8;118:9-20. doi: 10.1016/j.ejmech.2016.04.045. Epub 2016 Apr 19. Eur J Med Chem. 2016. PMID: 27116710
-
Role of JNK and NF-κB in mediating the effect of combretastatin A-4 and brimamin on endothelial and carcinoma cells.Cell Oncol (Dordr). 2015 Dec;38(6):463-78. doi: 10.1007/s13402-015-0243-7. Epub 2015 Sep 10. Cell Oncol (Dordr). 2015. PMID: 26358135
-
Combretastatin-based compounds with therapeutic characteristics: a patent review.Expert Opin Ther Pat. 2019 Sep;29(9):703-731. doi: 10.1080/13543776.2019.1651841. Epub 2019 Aug 8. Expert Opin Ther Pat. 2019. PMID: 31369715 Review.
-
Recent Trends in Tubulin-Binding Combretastatin A-4 Analogs for Anticancer Drug Development.Curr Med Chem. 2022;29(21):3748-3773. doi: 10.2174/0929867328666211202101641. Curr Med Chem. 2022. PMID: 34856892 Review.
Cited by
-
Luciferase Expressing Preclinical Model Systems Representing the Different Molecular Subtypes of Colorectal Cancer.Cancers (Basel). 2023 Aug 16;15(16):4122. doi: 10.3390/cancers15164122. Cancers (Basel). 2023. PMID: 37627150 Free PMC article.
References
-
- Siemann D.W., Bibby M.C., Dark G.G., Dicker A.P., Eskens F.A., Horsman M.R., Marme D., Lorusso P.M. Differentiation and definition of vascular-targeted therapies. Clin. Cancer Res. 2005;11:416–420. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

