Protective Effects of Glutamine and Leucine Supplementation on Sepsis-Induced Skeletal Muscle Injuries
- PMID: 34884807
- PMCID: PMC8657647
- DOI: 10.3390/ijms222313003
Protective Effects of Glutamine and Leucine Supplementation on Sepsis-Induced Skeletal Muscle Injuries
Abstract
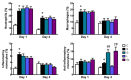
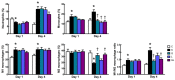
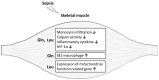
This study investigated the effects of l-glutamine (Gln) and/or l-leucine (Leu) administration on sepsis-induced skeletal muscle injuries. C57BL/6J mice were subjected to cecal ligation and puncture to induce polymicrobial sepsis and then given an intraperitoneal injection of Gln, Leu, or Gln plus Leu beginning at 1 h after the operation with re-injections every 24 h. All mice were sacrificed on either day 1 or day 4 after the operation. Blood and muscles were collected for analysis of inflammation and oxidative damage-related biomolecules. Results indicated that both Gln and Leu supplementation alleviated sepsis-induced skeletal muscle damage by reducing monocyte infiltration, calpain activity, and mRNA expression levels of inflammatory cytokines and hypoxia-inducible factor-1α. Furthermore, septic mice treated with Gln had higher percentages of blood anti-inflammatory monocytes and muscle M2 macrophages, whereas Leu treatment enhanced the muscle expressions of mitochondrion-related genes. However, there were no synergistic effects when Gln and Leu were simultaneously administered. These findings suggest that both Gln and Leu had prominent abilities to attenuate inflammation and degradation of skeletal muscles in the early and/or late phases of sepsis. Moreover, Gln promoted the switch of leukocytes toward an anti-inflammatory phenotype, while Leu treatment maintained muscle bioenergetic function.
Keywords: calpain; hypoxia-inducible factor-1α; macrophage; mitochondria; monocyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
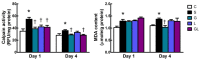
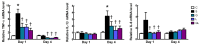
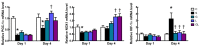

Similar articles
-
Glutamine and leucine administration attenuates muscle atrophy in sepsis.Life Sci. 2023 Feb 1;314:121327. doi: 10.1016/j.lfs.2022.121327. Epub 2022 Dec 27. Life Sci. 2023. PMID: 36584912
-
Oral free and dipeptide forms of glutamine supplementation attenuate oxidative stress and inflammation induced by endotoxemia.Nutrition. 2014 May;30(5):602-11. doi: 10.1016/j.nut.2013.10.019. Epub 2013 Oct 31. Nutrition. 2014. PMID: 24698353
-
Glutamine Administration Attenuates Kidney Inflammation in Obese Mice Complicated with Polymicrobial Sepsis.Mediators Inflamm. 2021 Mar 30;2021:5597118. doi: 10.1155/2021/5597118. eCollection 2021. Mediators Inflamm. 2021. PMID: 33859538 Free PMC article.
-
Glutamine Administration in Early or Late Septic Phase Downregulates Lymphocyte PD-1/PD-L1 Expression and the Inflammatory Response in Mice With Polymicrobial Sepsis.JPEN J Parenter Enteral Nutr. 2018 Mar;42(3):538-549. doi: 10.1177/0148607117695245. Epub 2017 Dec 12. JPEN J Parenter Enteral Nutr. 2018. PMID: 28633555
-
Effects of dietary glutamine supplementation on immune cell polarization and muscle regeneration in diabetic mice with limb ischemia.Eur J Nutr. 2020 Apr;59(3):921-933. doi: 10.1007/s00394-019-01951-4. Epub 2019 May 7. Eur J Nutr. 2020. PMID: 31062080
Cited by
-
A scoping review of preclinical intensive care unit-acquired weakness models.Front Physiol. 2024 Oct 2;15:1423567. doi: 10.3389/fphys.2024.1423567. eCollection 2024. Front Physiol. 2024. PMID: 39416383 Free PMC article.
-
Identification of ferroptosis-associated genes and potential pharmacological targets in sepsis-induced myopathy.Heliyon. 2024 Mar 30;10(7):e29062. doi: 10.1016/j.heliyon.2024.e29062. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38601693 Free PMC article.
-
Lipid droplets-related Perilipin-3: potential immune checkpoint and oncogene in oral squamous cell carcinoma.Cancer Immunol Immunother. 2024 Mar 30;73(5):78. doi: 10.1007/s00262-024-03659-9. Cancer Immunol Immunother. 2024. PMID: 38554152 Free PMC article.
-
The role of amino acid metabolism in inflammatory bowel disease and other inflammatory diseases.Front Immunol. 2023 Oct 23;14:1284133. doi: 10.3389/fimmu.2023.1284133. eCollection 2023. Front Immunol. 2023. PMID: 37936710 Free PMC article. Review.
-
Nutritional Biochemistry.Int J Mol Sci. 2023 Jun 2;24(11):9661. doi: 10.3390/ijms24119661. Int J Mol Sci. 2023. PMID: 37298610 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

