Lithium chloride inhibits infectious bronchitis virus-induced apoptosis and inflammation
- PMID: 34883226
- PMCID: PMC8648602
- DOI: 10.1016/j.micpath.2021.105352
Lithium chloride inhibits infectious bronchitis virus-induced apoptosis and inflammation
Abstract
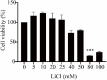
Avian infectious bronchitis (IB) was caused by infectious bronchitis virus (IBV), a coronavirus, which leads to enormous economic losses in the poultry industry. Studies have shown that lithium chloride (LiCl) is a good virus inhibitor. Through cell culture, virus infection, and RT-qPCR, we found that LiCl could down-regulate the apoptosis-related genes Caspase-3 and Bax, up-regulate Bcl-2, and down-regulate the inflammatory-related genes (NF-κB, NLRP3, TNF-α, and IL-1β) via inhibiting virus replication. Finally, clinical trials showed that LiCl could inhibit IBV-induced apoptosis and inflammatory in chicken embryos as well as reduce the mortality and deformity rate of chicken embryos. The results showed that LiCl has antiviral activity against IBV and clinical effects. Further studies are required to explore the exact action mechanism of LiCl on IBV-induced apoptosis and inflammation.
Keywords: Apoptosis; Chicken embryos; IBV; Inflammation; LiCl; Survival rate.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Andrographolide inhibits infectious bronchitis virus-induced apoptosis, pyroptosis, and inflammation.Antivir Ther. 2023 Oct;28(5):13596535231207499. doi: 10.1177/13596535231207499. Antivir Ther. 2023. PMID: 37846668
-
Lithium chloride inhibits the coronavirus infectious bronchitis virus in cell culture.Avian Pathol. 2007 Apr;36(2):109-14. doi: 10.1080/03079450601156083. Avian Pathol. 2007. PMID: 17479370 Free PMC article.
-
Astragalus polysaccharides inhibit avian infectious bronchitis virus infection by regulating viral replication.Microb Pathog. 2018 Jan;114:124-128. doi: 10.1016/j.micpath.2017.11.026. Epub 2017 Nov 21. Microb Pathog. 2018. PMID: 29170045 Free PMC article.
-
Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus.Avian Pathol. 2003 Dec;32(6):567-82. doi: 10.1080/03079450310001621198. Avian Pathol. 2003. PMID: 14676007 Free PMC article. Review.
-
Avian infectious bronchitis virus.Rev Sci Tech. 2000 Aug;19(2):493-508. doi: 10.20506/rst.19.2.1228. Rev Sci Tech. 2000. PMID: 10935276 Review.
Cited by
-
A Protective Role of Canonical Wnt/β-Catenin Pathway in Pathogenic Bacteria-Induced Inflammatory Responses.Mediators Inflamm. 2024 Feb 27;2024:8869510. doi: 10.1155/2024/8869510. eCollection 2024. Mediators Inflamm. 2024. PMID: 38445290 Free PMC article. Review.
References
-
- J M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;4(56):634–641. - PubMed
-
- Ramakrishnan S., Kappala D. Avian Infectious Bronchitis Virus. 2019:301–319.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

