Efficacy and safety of current treatment interventions for patients with severe COVID-19 infection: A network meta-analysis of randomized controlled trials
- PMID: 34882805
- PMCID: PMC9015508
- DOI: 10.1002/jmv.27512
Efficacy and safety of current treatment interventions for patients with severe COVID-19 infection: A network meta-analysis of randomized controlled trials
Abstract
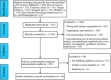
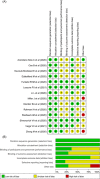
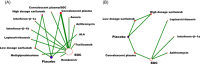
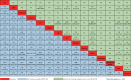
This study aimed to assess the efficacy and safety of different medications available at present for severe coronavirus disease 2019 (COVID-19) infection. We searched databases for randomized controlled trials (RCTs) published up to April 30, 2021, with Chinese or English language restriction, of medications recommended for patients (aged 18 years or older) with severe COVID-19 infection. We extracted data on trials and patient characteristics, and the following primary outcomes: all-cause mortality (ACM), and treatment-emergent adverse events (TEAEs). We identified 1855 abstracts and of these included 15 RCTs comprising 3073 participants through database searches and other sources. In terms of efficacy, compared with the standard of care (SOC) group, no significant decrease in ACM was found in α-lipoic acid, convalescent plasma (CP), azithromycin, tocilizumab, methylprednisolone, interferon beta, CP/SOC, high dosage sarilumab, low dosage sarilumab, remdesivir, lopinavir-ritonavir, auxora, and placebo group. Compared with placebo, we found that a significant decrease in ACM was only found in methylprednisolone (odds ratio [OR]: 0.16, 95% confidence interval [CI]: 0.03-0.75]. With respect to TEAEs, the CP group showed lower TEAEs than placebo (OR: 0.07, 95% CI: 0.01-0.58) or SOC (OR: 0.05, 95% CI: 0.01-0.42) group for the therapy of severe COVID-19 patients. This study only demonstrated that methylprednisolone was superior to placebo in treating patients with severe COVID-19 infection. Meanwhile, this further confirmed that the safety of other treatment interventions might be inferior to CP for the therapy of severe COVID-19 patients.
Keywords: efficacy; network meta-analysis; randomized controlled trials; safety; severe COVID-19.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

Similar articles
-
Comparative efficacy and safety of pharmacological interventions for severe COVID-19 patients: An updated network meta-analysis of 48 randomized controlled trials.Medicine (Baltimore). 2022 Oct 14;101(41):e30998. doi: 10.1097/MD.0000000000030998. Medicine (Baltimore). 2022. PMID: 36254081 Free PMC article.
-
Efficacy and safety of current medications for treating severe and non-severe COVID-19 patients: an updated network meta-analysis of randomized placebo-controlled trials.Aging (Albany NY). 2021 Sep 16;13(18):21866-21902. doi: 10.18632/aging.203522. Epub 2021 Sep 16. Aging (Albany NY). 2021. PMID: 34531332 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Comparative Effectiveness of Pharmacological Interventions for Covid-19: A Systematic Review and Network Meta-Analysis.Front Pharmacol. 2021 May 3;12:649472. doi: 10.3389/fphar.2021.649472. eCollection 2021. Front Pharmacol. 2021. PMID: 34012398 Free PMC article. Review.
-
The Standard of Care Definitions on COVID-19 Pharmacological Clinical Trials: A Systematic Review.Front Pharmacol. 2021 Oct 18;12:749514. doi: 10.3389/fphar.2021.749514. eCollection 2021. Front Pharmacol. 2021. PMID: 34733161 Free PMC article. Review.
Cited by
-
Natural inhibitors of SARS-CoV-2 main protease: structure based pharmacophore modeling, molecular docking and molecular dynamic simulation studies.J Mol Model. 2022 Aug 29;28(9):279. doi: 10.1007/s00894-022-05286-6. J Mol Model. 2022. PMID: 36031629 Free PMC article.
-
Delta neutrophil index and C-reactive protein: a potential diagnostic marker of multisystem inflammatory syndrome in children (MIS-C) with COVID-19.Eur J Pediatr. 2022 Feb;181(2):775-781. doi: 10.1007/s00431-021-04281-y. Epub 2021 Oct 14. Eur J Pediatr. 2022. PMID: 34647164 Free PMC article.
-
Clinical prediction models in hospitalized patients with COVID-19: A multicenter cohort study.Respir Med. 2022 Oct;202:106954. doi: 10.1016/j.rmed.2022.106954. Epub 2022 Aug 21. Respir Med. 2022. PMID: 36057141 Free PMC article.
-
High-cited favorable studies for COVID-19 treatments ineffective in large trials.J Clin Epidemiol. 2022 Aug;148:1-9. doi: 10.1016/j.jclinepi.2022.04.001. Epub 2022 Apr 6. J Clin Epidemiol. 2022. PMID: 35398190 Free PMC article.
References
-
- World Health Organization . WHO coronavirus (COVID‐19) dashboard. Geneva: WHO; 2020. Accessed May 7, 2021. https://covid19.who.int/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

