Efficacy and safety of a thermosensitive hydrogel for endoscopic submucosal dissection: An in vivo swine study
- PMID: 34882721
- PMCID: PMC8659419
- DOI: 10.1371/journal.pone.0260458
Efficacy and safety of a thermosensitive hydrogel for endoscopic submucosal dissection: An in vivo swine study
Abstract
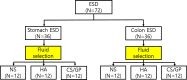
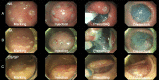
Injectable thermo-sensitive chitosan hydrogels have recently been developed for the use of submucosal fluids in endoscopic submucosal dissections (ESD). This study aimed to investigate the efficacy and safety of chitosan hydrogels during ESD. Submucosal fluids were administered as follows: 0.9% normal saline (NS), 0.4% hyaluronic acid (HA) and chitosan/β-glycerophosphate (CS/GP) hydrogel. Each solution was administered twice into the stomach and colon of a pig, with a total of 72 ESD procedures performed on 12 pigs. The injected volume and procedure-related parameters were recorded and analyzed. ESDs that created ulcers after 7 days were histologically compared. All ESD specimens were resected en bloc. The total injected volumes during ESD of the stomach (NS, 16.09±3.27 vs. HA, 11.17±2.32 vs. CS/GP, 9.44±2.33; p<0.001) and colon (NS, 9.17±1.80 vs. HA, 6.67±1.50 vs. CS/GP, 6.75±1.57; p = 0.001) were significantly different. Hydrogel showed significant differences from normal saline in terms of fluid power (mm2/vol; NS, 35.70±9.00 vs. CS/GP 57.48±20.77; p = 0.001) and consumption rate (vol/min; NS, 2.59±0.86 vs. CS/GP, 1.62±0.65; p = 0.013) in the stomach. Histological examination revealed preserved muscularis propria, although the chitosan hydrogel resulted in a partial inflammatory response, with a hypertrophied submucosal layer. Chitosan hydrogel was found to be superior to normal saline, with an efficacy similar to that of hyaluronic acid. Nonetheless, long-term histological changes should be evaluated before clinical implementation.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Chitosan thermosensitive hydrogels based on lyophilizate powders demonstrate significant potential for clinical use in endoscopic submucosal dissection procedures.Int J Biol Macromol. 2021 Aug 1;184:593-603. doi: 10.1016/j.ijbiomac.2021.06.111. Epub 2021 Jun 24. Int J Biol Macromol. 2021. PMID: 34174301
-
Design and validation of performance-oriented injectable chitosan thermosensitive hydrogels for endoscopic submucosal dissection.Biomater Adv. 2023 Mar;146:213286. doi: 10.1016/j.bioadv.2023.213286. Epub 2023 Jan 13. Biomater Adv. 2023. PMID: 36657218
-
Injectable chitosan-polyvinylpyrrolidone composite thermosensitive hydrogels with sustained submucosal lifting for endoscopic submucosal dissection.Int J Biol Macromol. 2024 Sep;276(Pt 1):133165. doi: 10.1016/j.ijbiomac.2024.133165. Epub 2024 Jun 18. Int J Biol Macromol. 2024. PMID: 38901518
-
Injectable thermosensitive chitosan/glycerophosphate-based hydrogels for tissue engineering and drug delivery applications: a review.Recent Pat Drug Deliv Formul. 2015;9(2):107-20. doi: 10.2174/1872211308666141028145651. Recent Pat Drug Deliv Formul. 2015. PMID: 25354269 Review.
-
A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration.Int J Biol Macromol. 2019 Jan;121:38-54. doi: 10.1016/j.ijbiomac.2018.10.014. Epub 2018 Oct 4. Int J Biol Macromol. 2019. PMID: 30291931 Review.
References
-
- Mehta N, Strong AT, Franco M, Stevens T, Chahal P, Jang S, et al.. Optimal injection solution for endoscopic submucosal dissection: A randomized controlled trial of Western solutions in a porcine model. Digestive endoscopy: official journal of the Japan Gastroenterological Endoscopy Society. 2018;30(3):347–53. doi: 10.1111/den.12993 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

