Comparative efficacy of secukinumab against adalimumab and infliximab in patients with moderate-to-severe plaque psoriasis
- PMID: 34882622
- PMCID: PMC8850822
- DOI: 10.1097/CM9.0000000000001817
Comparative efficacy of secukinumab against adalimumab and infliximab in patients with moderate-to-severe plaque psoriasis
Abstract
Background: Psoriasis is a common, chronic, immune-mediated inflammatory skin disease with increased epidermal proliferation. The objective of this review was to systematically identify the evidence and perform a network meta-analysis (NMA) to estimate the relative efficacy of secukinumab (SEC) against adalimumab (ADA) and infliximab (INF) for the treatment of moderate-to-severe plaque psoriasis.
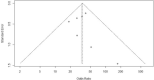
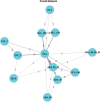
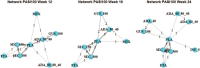
Methods: A systematic literature review (SLR) was conducted according to a pre-specified protocol to identify relevant studies. Initially, the databases were searched from database inception till June 2013, and the SLR was updated in April 2020. The eligibility criteria included adult patients (≥18 years old) with moderate-to-severe plaque psoriasis, and the SLR included randomized controlled trials (RCTs). The comparators of interest were SEC, ADA, INF, and placebo (PLA), while outcomes of interest were Psoriasis Area and Severity Index (PASI) (50, 75, and 90) at weeks 12, 16, and 24. A Bayesian NMA for PASI was utilized with a framework that evaluated the probability of PASI responses in different categories of PASI thresholds within a single model.
Results: A total of 23 RCTs that assessed the efficacy of SEC, ADA, and INF in patients with moderate-to-severe plaque psoriasis were identified. At 12 weeks, SEC was associated with a significantly better response compared with PLA and ADA for PASI 75 and 90, while response results were comparable against INF. At 12 weeks, risk ratio (95% confidence interval) derived from NMA for SEC vs. ADA and INF for PASI 75 was 1.35 (1.19, 1.57) and 1.01 (0.90, 1.18), respectively. At the 16-week and 24-week time interval, SEC was significantly better than PLA, ADA, and INF for PASI 75 and 90.
Conclusion: Efficacy of SEC in the treatment of patient populations with moderate-to-severe plaque psoriasis is well demonstrated through NMA.
Copyright © 2021 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Figures

Similar articles
-
Efficacy of Brodalumab for Moderate to Severe Plaque Psoriasis: A Canadian Network Meta-Analysis.J Cutan Med Surg. 2020 Nov/Dec;24(6):561-572. doi: 10.1177/1203475420933174. Epub 2020 Jun 26. J Cutan Med Surg. 2020. PMID: 32588642
-
Decision-Analytic Modeling for Time-Effectiveness of the Sequence of Induction Treatments for Moderate to Severe Plaque Psoriasis.JAMA Dermatol. 2019 Dec 1;155(12):1380-1389. doi: 10.1001/jamadermatol.2019.2941. JAMA Dermatol. 2019. PMID: 31617856 Free PMC article.
-
Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response.J Eur Acad Dermatol Venereol. 2019 Feb;33(2):355-366. doi: 10.1111/jdv.15277. Epub 2018 Oct 31. J Eur Acad Dermatol Venereol. 2019. PMID: 30289198 Free PMC article.
-
Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials.Br J Dermatol. 2008 Sep;159(3):513-26. doi: 10.1111/j.1365-2133.2008.08732.x. Epub 2008 Jul 9. Br J Dermatol. 2008. PMID: 18627372 Review.
-
Efficacy of biologics in the treatment of moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials with different time points.J Eur Acad Dermatol Venereol. 2014 Dec;28(12):1633-53. doi: 10.1111/jdv.12238. Epub 2013 Aug 19. J Eur Acad Dermatol Venereol. 2014. PMID: 24033851 Review.
Cited by
-
The PI3K-Akt-mTOR and Associated Signaling Pathways as Molecular Drivers of Immune-Mediated Inflammatory Skin Diseases: Update on Therapeutic Strategy Using Natural and Synthetic Compounds.Cells. 2023 Jun 20;12(12):1671. doi: 10.3390/cells12121671. Cells. 2023. PMID: 37371141 Free PMC article. Review.
-
Childhood guttate psoriasis: an updated review.Drugs Context. 2023 Oct 23;12:2023-8-2. doi: 10.7573/dic.2023-8-2. eCollection 2023. Drugs Context. 2023. PMID: 37908643 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Jul 12;7(7):CD011535. doi: 10.1002/14651858.CD011535.pub6. Cochrane Database Syst Rev. 2023. PMID: 37436070 Free PMC article. Review.
-
Cost-Effectiveness of Secukinumab Versus Other Biologics in the Treatment of Moderate-to-Severe Plaque Psoriasis: The Chinese Healthcare System Perspective.Dermatol Ther (Heidelb). 2023 Nov;13(11):2681-2696. doi: 10.1007/s13555-023-01041-8. Epub 2023 Sep 23. Dermatol Ther (Heidelb). 2023. PMID: 37741954 Free PMC article.
-
Efficacy and safety of secukinumab in Chinese patients with psoriatic arthritis: Evidence from a real-world study.Chin Med J (Engl). 2024 Nov 5;137(21):2618-2620. doi: 10.1097/CM9.0000000000003316. Epub 2024 Sep 23. Chin Med J (Engl). 2024. PMID: 39307939 Free PMC article. No abstract available.
References
-
- Chen XL, Zheng LY, Zhang H, Zhang JZ, Zhang CL, Ju M, et al. . Disease burden and quality of life in patients with psoriasis: an internet based questionnaire survey (in Chinese). Chin J Dermatol 2019; 52:791–795. doi: 10.35541/cjd.20190247.
-
- Committee on Psoriasis, Chinese Society of Dermatology. Guideline for the diagnosis and treatment of psoriasis in China (2018 complete edition) (in Chinese). Chin J Dermatol 2019; 52:667–710. doi: 10.35541/cjd.20190847.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

