TTK inhibition increases cisplatin sensitivity in high-grade serous ovarian carcinoma through the mTOR/autophagy pathway
- PMID: 34876569
- PMCID: PMC8651821
- DOI: 10.1038/s41419-021-04429-6
TTK inhibition increases cisplatin sensitivity in high-grade serous ovarian carcinoma through the mTOR/autophagy pathway
Abstract
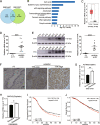
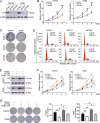
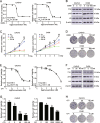
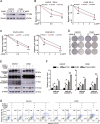
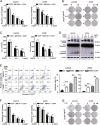
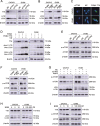
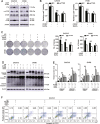
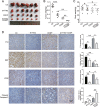
High-grade serous ovarian cancer (HGSOC) is the most lethal gynecological malignancy. However, the molecular mechanisms underlying HGSOC development, progression, chemotherapy insensitivity and resistance remain unclear. Two independent GEO datasets, including the gene expression profile of primary ovarian carcinoma and normal controls, were analyzed to identify genes related to HGSOC development and progression. A KEGG pathway analysis of the differentially expressed genes (DEGs) revealed that the cell cycle pathway was the most enriched pathway, among which TTK protein kinase (TTK) was the only gene with a clinical-grade inhibitor that has been investigated in a clinical trial but had not been studied in HGSOC. TTK was also upregulated in cisplatin-resistant ovarian cancer cells from two other datasets. TTK is a regulator of spindle assembly checkpoint signaling, playing an important role in cell cycle control and tumorigenesis in various cancers. However, the function and regulatory mechanism of TTK in HGSOC remain to be determined. In this study, we observed TTK upregulation in patients with HGSOC. High TTK expression was related to a poor prognosis. Genetic and pharmacological inhibition of TTK impeded the proliferation of ovarian cancer cells by disturbing cell cycle progression and increasing apoptosis. TTK silencing increased cisplatin sensitivity by activating the mammalian target of rapamycin (mTOR) complex to further suppress cisplatin-induced autophagy in vitro. In addition, the enhanced sensitivity was partially diminished by rapamycin-mediated inhibition of mTOR in TTK knockdown cells. Furthermore, TTK knockdown increased the toxicity of cisplatin in vivo by decreasing autophagy. These findings suggest that the administration of TTK inhibitors in combination with cisplatin may lead to improved response rates to cisplatin in patients with HGSOC presenting high TTK expression. In summary, our study may provide a theoretical foundation for using the combination therapy of cisplatin and TTK inhibitors as a treatment for HGSOC in the future.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
PBK, targeted by EVI1, promotes metastasis and confers cisplatin resistance through inducing autophagy in high-grade serous ovarian carcinoma.Cell Death Dis. 2019 Feb 18;10(3):166. doi: 10.1038/s41419-019-1415-6. Cell Death Dis. 2019. PMID: 30778048 Free PMC article.
-
TTK is a potential therapeutic target for cisplatin-resistant ovarian cancer.J Ovarian Res. 2021 Oct 2;14(1):128. doi: 10.1186/s13048-021-00884-z. J Ovarian Res. 2021. PMID: 34598710 Free PMC article.
-
ROR2 induces cell apoptosis via activating IRE1α/JNK/CHOP pathway in high-grade serous ovarian carcinoma in vitro and in vivo.J Transl Med. 2019 Dec 26;17(1):428. doi: 10.1186/s12967-019-02178-x. J Transl Med. 2019. PMID: 31878941 Free PMC article.
-
Targeting the PI3K/mTOR Pathway Augments CHK1 Inhibitor-Induced Replication Stress and Antitumor Activity in High-Grade Serous Ovarian Cancer.Cancer Res. 2020 Dec 1;80(23):5380-5392. doi: 10.1158/0008-5472.CAN-20-1439. Epub 2020 Sep 30. Cancer Res. 2020. PMID: 32998994 Free PMC article.
-
Inhibition of apoptosis through AKT-mTOR pathway in ovarian cancer and renal cancer.Aging (Albany NY). 2023 Feb 27;15(4):1210-1227. doi: 10.18632/aging.204564. Epub 2023 Feb 27. Aging (Albany NY). 2023. PMID: 36849137 Free PMC article.
Cited by
-
Dual TTK/PLK1 inhibition has potent anticancer activity in TNBC as monotherapy and in combination.Front Oncol. 2024 Aug 9;14:1447807. doi: 10.3389/fonc.2024.1447807. eCollection 2024. Front Oncol. 2024. PMID: 39184047 Free PMC article.
-
Role of autophagy and its regulation by noncoding RNAs in ovarian cancer.Exp Biol Med (Maywood). 2023 Jun;248(12):1001-1012. doi: 10.1177/15353702231151958. Epub 2023 Feb 18. Exp Biol Med (Maywood). 2023. PMID: 36803116 Free PMC article. Review.
-
Bibliometric and visualized analysis of drug resistance in ovarian cancer from 2013 to 2022.Front Oncol. 2023 May 18;13:1173863. doi: 10.3389/fonc.2023.1173863. eCollection 2023. Front Oncol. 2023. PMID: 37324006 Free PMC article. Review.
-
PhenoMultiOmics: an enzymatic reaction inferred multi-omics network visualization web server.Bioinformatics. 2024 Nov 1;40(11):btae623. doi: 10.1093/bioinformatics/btae623. Bioinformatics. 2024. PMID: 39418180 Free PMC article.
-
Annexin A2 combined with TTK accelerates esophageal cancer progression via the Akt/mTOR signaling pathway.Cell Death Dis. 2024 Apr 24;15(4):291. doi: 10.1038/s41419-024-06683-w. Cell Death Dis. 2024. PMID: 38658569 Free PMC article.
References
-
- Armstrong D, Alvarez R, Bakkum-Gamez J, Barroilhet L, Behbakht K, Berchuck A, et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19:191–226. - PubMed
-
- Bookman M. Optimal primary therapy of ovarian cancer. Ann Oncol. 2016;27:i58–i62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

