Gram-negative bacteria and their lipopolysaccharides in Alzheimer's disease: pathologic roles and therapeutic implications
- PMID: 34876226
- PMCID: PMC8650380
- DOI: 10.1186/s40035-021-00273-y
Gram-negative bacteria and their lipopolysaccharides in Alzheimer's disease: pathologic roles and therapeutic implications
Abstract
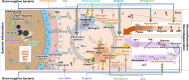
Alzheimer's disease (AD) is the most serious age-related neurodegenerative disease and causes destructive and irreversible cognitive decline. Failures in the development of therapeutics targeting amyloid-β (Aβ) and tau, principal proteins inducing pathology in AD, suggest a paradigm shift towards the development of new therapeutic targets. The gram-negative bacteria and lipopolysaccharides (LPS) are attractive new targets for AD treatment. Surprisingly, an altered distribution of gram-negative bacteria and their LPS has been reported in AD patients. Moreover, gram-negative bacteria and their LPS have been shown to affect a variety of AD-related pathologies, such as Aβ homeostasis, tau pathology, neuroinflammation, and neurodegeneration. Moreover, therapeutic approaches targeting gram-negative bacteria or gram-negative bacterial molecules have significantly alleviated AD-related pathology and cognitive dysfunction. Despite multiple evidence showing that the gram-negative bacteria and their LPS play a crucial role in AD pathogenesis, the pathogenic mechanisms of gram-negative bacteria and their LPS have not been clarified. Here, we summarize the roles and pathomechanisms of gram-negative bacteria and LPS in AD. Furthermore, we discuss the possibility of using gram-negative bacteria and gram-negative bacterial molecules as novel therapeutic targets and new pathological characteristics for AD.
Keywords: Alzheimer’s disease; Amyloid beta; Exotoxin; Gram-negative bacteria; Lipopolysaccharide; Tau.
© 2021. The Author(s).
Conflict of interest statement
Authors declare no competing interests.
Figures



Similar articles
-
The endotoxin hypothesis of Alzheimer's disease.Mol Neurodegener. 2024 Apr 1;19(1):30. doi: 10.1186/s13024-024-00722-y. Mol Neurodegener. 2024. PMID: 38561809 Free PMC article. Review.
-
Gram-negative bacterial molecules associate with Alzheimer disease pathology.Neurology. 2016 Nov 29;87(22):2324-2332. doi: 10.1212/WNL.0000000000003391. Epub 2016 Oct 26. Neurology. 2016. PMID: 27784770 Free PMC article.
-
Human gut microbiota Agathobaculum butyriciproducens improves cognitive impairment in LPS-induced and APP/PS1 mouse models of Alzheimer's disease.Nutr Res. 2021 Feb;86:96-108. doi: 10.1016/j.nutres.2020.12.010. Epub 2020 Dec 9. Nutr Res. 2021. PMID: 33551257
-
Ghrelin in Alzheimer's disease: Pathologic roles and therapeutic implications.Ageing Res Rev. 2019 Nov;55:100945. doi: 10.1016/j.arr.2019.100945. Epub 2019 Aug 18. Ageing Res Rev. 2019. PMID: 31434007 Review.
-
The interactions of p53 with tau and Aß as potential therapeutic targets for Alzheimer's disease.Prog Neurobiol. 2018 Sep;168:104-127. doi: 10.1016/j.pneurobio.2018.05.001. Epub 2018 May 4. Prog Neurobiol. 2018. PMID: 29733887 Review.
Cited by
-
Modulation of Gut Microbiota Through Dietary Intervention in Neuroinflammation and Alzheimer's and Parkinson's Diseases.Curr Nutr Rep. 2024 Jun;13(2):82-96. doi: 10.1007/s13668-024-00539-7. Epub 2024 Apr 23. Curr Nutr Rep. 2024. PMID: 38652236 Free PMC article. Review.
-
Impact of Microbiome-Brain Communication on Neuroinflammation and Neurodegeneration.Int J Mol Sci. 2023 Oct 5;24(19):14925. doi: 10.3390/ijms241914925. Int J Mol Sci. 2023. PMID: 37834373 Free PMC article. Review.
-
Gut microbiota-host lipid crosstalk in Alzheimer's disease: implications for disease progression and therapeutics.Mol Neurodegener. 2024 Apr 16;19(1):35. doi: 10.1186/s13024-024-00720-0. Mol Neurodegener. 2024. PMID: 38627829 Free PMC article. Review.
-
Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders.Metabolites. 2022 Nov 3;12(11):1064. doi: 10.3390/metabo12111064. Metabolites. 2022. PMID: 36355147 Free PMC article. Review.
-
The mechanisms of Porphyromonas gingivalis-derived outer membrane vesicles-induced neurotoxicity and microglia activation.J Dent Sci. 2024 Jul;19(3):1434-1442. doi: 10.1016/j.jds.2024.04.002. Epub 2024 Apr 17. J Dent Sci. 2024. PMID: 39035337 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

