The germinal centre B cell response to SARS-CoV-2
- PMID: 34873279
- PMCID: PMC8647067
- DOI: 10.1038/s41577-021-00657-1
The germinal centre B cell response to SARS-CoV-2
Abstract
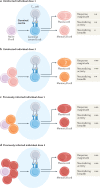
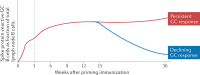
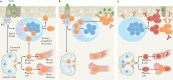
The germinal centre (GC) response is critical for the generation of affinity-matured plasma cells and memory B cells capable of mediating long-term protective immunity. Understanding whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or vaccination elicits a GC response has profound implications for the capacity of responding B cells to contribute to protection against infection. However, direct assessment of the GC response in humans remains a major challenge. Here we summarize emerging evidence for the importance of the GC response in the establishment of durable and broad immunity against SARS-CoV-2 and discuss new approaches to modulate the GC response to better protect against newly emerging SARS-CoV-2 variants. We also discuss new findings showing that the GC B cell response persists in the draining lymph nodes for at least 6 months in some individuals following vaccination with SARS-CoV-2 mRNA-based vaccines.
© 2021. Springer Nature Limited.
Conflict of interest statement
The Ellebedy laboratory received funding under sponsored research agreements that are unrelated to the data presented in the current study from Emergent BioSolutions and from AbbVie. A.H.E. has received consulting payments from Mubadala Investment Company, InBios International LLC and Fimbrion Therapeutics and is the founder of ImmuneBio Consulting LLC. B.J.L. declares no competing interests.
Figures


Similar articles
-
SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses.Nature. 2021 Aug;596(7870):109-113. doi: 10.1038/s41586-021-03738-2. Epub 2021 Jun 28. Nature. 2021. PMID: 34182569 Free PMC article.
-
What the Omicron wave is revealing about human immunity.Nature. 2022 Feb;602(7895):22-25. doi: 10.1038/d41586-022-00214-3. Nature. 2022. PMID: 35110764 No abstract available.
-
Ipsilateral or contralateral boosting of mice with mRNA vaccines confers equivalent immunity and protection against a SARS-CoV-2 Omicron strain.J Virol. 2024 Sep 17;98(9):e0057424. doi: 10.1128/jvi.00574-24. Epub 2024 Aug 28. J Virol. 2024. PMID: 39194250 Free PMC article.
-
Unravelling humoral immunity in SARS-CoV-2: Insights from infection and vaccination.Hum Antibodies. 2024;32(3):85-106. doi: 10.3233/HAB-230017. Hum Antibodies. 2024. PMID: 38758995 Review.
-
Follicular Helper T Cells in the Immunopathogenesis of SARS-CoV-2 Infection.Front Immunol. 2021 Sep 16;12:731100. doi: 10.3389/fimmu.2021.731100. eCollection 2021. Front Immunol. 2021. PMID: 34603308 Free PMC article. Review.
Cited by
-
Developing dendritic cell for SARS-CoV-2 vaccine: Breakthrough in the pandemic.Front Immunol. 2022 Sep 6;13:989685. doi: 10.3389/fimmu.2022.989685. eCollection 2022. Front Immunol. 2022. PMID: 36148241 Free PMC article. Review.
-
Homologous but not heterologous COVID-19 vaccine booster elicits IgG4+ B-cells and enhanced Omicron subvariant binding.NPJ Vaccines. 2024 Jul 17;9(1):129. doi: 10.1038/s41541-024-00919-8. NPJ Vaccines. 2024. PMID: 39013889 Free PMC article.
-
CaMK4 controls follicular helper T cell expansion and function during normal and autoimmune T-dependent B cell responses.Nat Commun. 2024 Jan 29;15(1):840. doi: 10.1038/s41467-024-45080-x. Nat Commun. 2024. PMID: 38287012 Free PMC article.
-
Immune responses in mildly versus critically ill COVID-19 patients.Front Immunol. 2023 Jan 30;14:1077236. doi: 10.3389/fimmu.2023.1077236. eCollection 2023. Front Immunol. 2023. PMID: 36793739 Free PMC article. Review.
-
Mechanistic Insights Into the Immune Pathophysiology of COVID-19; An In-Depth Review.Front Immunol. 2022 Mar 24;13:835104. doi: 10.3389/fimmu.2022.835104. eCollection 2022. Front Immunol. 2022. PMID: 35401519 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

