Corticotropin releasing hormone promotes inflammatory bowel disease via inducing intestinal macrophage autophagy
- PMID: 34873177
- PMCID: PMC8648763
- DOI: 10.1038/s41420-021-00767-8
Corticotropin releasing hormone promotes inflammatory bowel disease via inducing intestinal macrophage autophagy
Abstract
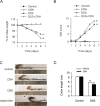
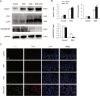
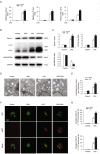
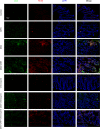
Psychosocial stress is a vital factor contributing to the pathogenesis and progression of inflammatory bowel disease (IBD). The contribution of intestinal macrophage autophagy to the onset and development of IBD has been widely studied. Herein, we investigated the underlying mechanism of psychosocial stress in an IBD mouse model pertaining to macrophage autophagy. Corticotropin releasing hormone (CRH) was peripherally administrated to induce psychosocial stress. For in vivo studies, dextran sulfate sodium (DSS) was used for the creation of our IBD mouse model. For in vitro studies, lipopolysaccharide (LPS) was applied on murine bone marrow-derived macrophages (BMDMs) as a cellular IBD-related challenge. Chloroquine was applied to inhibit autophagy. We found that CRH aggravated the severity of DSS-induced IBD, increasing overall and local inflammatory reactions and infiltration. The levels of autophagy in intestinal macrophages and murine BMDMs were increased under these IBD-related inflammatory challenges and CRH further enhanced these effects. Subsequent administration of chloroquine markedly attenuated the detrimental effects of CRH on IBD severity and inflammatory reactions via inhibition of autophagy. These findings illustrate the effects of peripheral administration of CRH on DSS-induced IBD via the enhancement of intestinal macrophage autophagy, thus providing a novel understanding as well as therapeutic target for the treatment of IBD.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease.Cell Death Dis. 2019 Sep 30;10(6):391. doi: 10.1038/s41419-019-1634-x. Cell Death Dis. 2019. PMID: 31564717 Free PMC article.
-
Alpha7 Nicotinic Acetylcholine Receptor Alleviates Inflammatory Bowel Disease Through Induction of AMPK-mTOR-p70S6K-Mediated Autophagy.Inflammation. 2019 Oct;42(5):1666-1679. doi: 10.1007/s10753-019-01027-9. Inflammation. 2019. PMID: 31236857
-
Identification of a novel interaction between corticotropin releasing hormone (Crh) and macroautophagy.Sci Rep. 2016 Mar 18;6:23342. doi: 10.1038/srep23342. Sci Rep. 2016. PMID: 26987580 Free PMC article.
-
Intestinal Macrophage Autophagy and its Pharmacological Application in Inflammatory Bowel Disease.Front Pharmacol. 2021 Nov 24;12:803686. doi: 10.3389/fphar.2021.803686. eCollection 2021. Front Pharmacol. 2021. PMID: 34899362 Free PMC article. Review.
-
Targeting macrophage autophagy for inflammation resolution and tissue repair in inflammatory bowel disease.Burns Trauma. 2023 May 4;11:tkad004. doi: 10.1093/burnst/tkad004. eCollection 2023. Burns Trauma. 2023. PMID: 37152076 Free PMC article. Review.
Cited by
-
Sleep-Disturbance-Induced Microglial Activation Involves CRH-Mediated Galectin 3 and Autophagy Dysregulation.Cells. 2022 Dec 30;12(1):160. doi: 10.3390/cells12010160. Cells. 2022. PMID: 36611953 Free PMC article.
-
Role of CRH in colitis and colitis-associated cancer: a combinative result of central and peripheral effects?Front Endocrinol (Lausanne). 2024 Mar 28;15:1363748. doi: 10.3389/fendo.2024.1363748. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38616821 Free PMC article. Review.
-
Vortioxetine as an alternative treatment for somatic symptom disorder: case report.Front Psychiatry. 2024 Nov 7;15:1496072. doi: 10.3389/fpsyt.2024.1496072. eCollection 2024. Front Psychiatry. 2024. PMID: 39575195 Free PMC article.
-
Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease.Front Endocrinol (Lausanne). 2023 Aug 28;14:1217165. doi: 10.3389/fendo.2023.1217165. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37701897 Free PMC article. Review.
-
Corticotropin-Releasing Hormone: Biology and Therapeutic Opportunities.Biology (Basel). 2022 Dec 8;11(12):1785. doi: 10.3390/biology11121785. Biology (Basel). 2022. PMID: 36552294 Free PMC article.
References
-
- Palmela C, Chevarin C, Xu Z, Torres J, Sevrin G, Hirten R, et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67:574–87.. - PubMed
-
- Singh S, Al-Darmaki A, Frolkis AD, Seow CH, Leung Y, Novak KL, et al. Postoperative mortality among patients with inflammatory bowel diseases: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2015;149:928–37. - PubMed
-
- Regueiro M, Greer JB, Szigethy E. Etiology and treatment of pain and psychosocial issues in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:430–9.e4. - PubMed
LinkOut - more resources
Full Text Sources

