Host directed therapies: COVID-19 and beyond
- PMID: 34870156
- PMCID: PMC8464038
- DOI: 10.1016/j.crphar.2021.100058
Host directed therapies: COVID-19 and beyond
Abstract
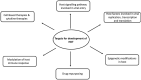
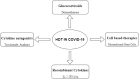
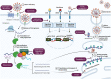
The global spread of SARS-CoV-2 has necessitated the development of novel, safe and effective therapeutic agents against this virus to stop the pandemic, however the development of novel antivirals may take years, hence, the best alternative available, is to repurpose the existing antiviral drugs with known safety profile in humans. After more than one year into this pandemic, global efforts have yielded the fruits and with the launch of many vaccines in the market, the world is inching towards the end of this pandemic, nonetheless, future pandemics of this magnitude or even greater cannot be denied. The preparedness against viruses of unknown origin should be maintained and the broad-spectrum antivirals with activity against range of viruses should be developed to curb future viral pandemics. The majority of antivirals developed till date are pathogen specific agents, which target critical viral pathways and lack broad spectrum activity required to target wide range of viruses. The surge in drug resistance among pathogens has rendered a compelling need to shift our focus towards host directed factors in the treatment of infectious diseases. This gains special relevance in the case of viral infections, where the pathogen encodes a handful of genes and predominantly depends on host factors for their propagation and persistence. Therefore, future antiviral drug development should focus more on targeting molecules of host pathways that are often hijacked by many viruses. Such cellular proteins of host pathways offer attractive targets for the development of broad-spectrum anticipatory antivirals. In the present article, we have reviewed the host directed therapies (HDTs) effective against viral infections with a special focus on COVID-19. This article also discusses the strategies involved in identifying novel host targets and subsequent development of broad spectrum HDTs.
Keywords: COVID-19; Host directed therapy; Human coronavirus; SARS-CoV-2.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Development of a Fluorescence-Based, High-Throughput SARS-CoV-2 3CLpro Reporter Assay.J Virol. 2020 Oct 27;94(22):e01265-20. doi: 10.1128/JVI.01265-20. Print 2020 Oct 27. J Virol. 2020. PMID: 32843534 Free PMC article.
-
Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine.Emerg Microbes Infect. 2020 Dec;9(1):2245-2255. doi: 10.1080/22221751.2020.1829082. Emerg Microbes Infect. 2020. PMID: 32975484 Free PMC article.
-
Host-directed therapies for bacterial and viral infections.Nat Rev Drug Discov. 2018 Jan;17(1):35-56. doi: 10.1038/nrd.2017.162. Epub 2017 Sep 22. Nat Rev Drug Discov. 2018. PMID: 28935918 Free PMC article. Review.
-
Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2.Protein Cell. 2020 Oct;11(10):723-739. doi: 10.1007/s13238-020-00768-w. Epub 2020 Aug 4. Protein Cell. 2020. PMID: 32754890 Free PMC article.
-
SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction.Int J Mol Sci. 2020 Jun 26;21(12):4549. doi: 10.3390/ijms21124549. Int J Mol Sci. 2020. PMID: 32604730 Free PMC article. Review.
Cited by
-
Bisacodyl Limits Chikungunya Virus Replication In Vitro and Is Broadly Antiviral.Antimicrob Agents Chemother. 2022 Jun 21;66(6):e0029222. doi: 10.1128/aac.00292-22. Epub 2022 Jun 2. Antimicrob Agents Chemother. 2022. PMID: 35652314 Free PMC article.
-
Single-stranded DNA oligonucleotides containing CpG motifs are non-stimulatory in vitro but offer protection in vivo against Burkholderia pseudomallei.Front Cell Infect Microbiol. 2024 Oct 18;14:1458435. doi: 10.3389/fcimb.2024.1458435. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39492991 Free PMC article.
-
An Antiherpesviral Host-Directed Strategy Based on CDK7 Covalently Binding Drugs: Target-Selective, Picomolar-Dose, Cross-Virus Reactivity.Pharmaceutics. 2024 Jan 23;16(2):158. doi: 10.3390/pharmaceutics16020158. Pharmaceutics. 2024. PMID: 38399219 Free PMC article.
-
Strategies Tackling Viral Replication and Inflammatory Pathways as Early Pharmacological Treatment for SARS-CoV-2 Infection: Any Potential Role for Ketoprofen Lysine Salt?Molecules. 2022 Dec 15;27(24):8919. doi: 10.3390/molecules27248919. Molecules. 2022. PMID: 36558048 Free PMC article. Review.
-
Identification of Dual Inhibitors Targeting Main Protease (Mpro) and Cathepsin L as Potential Anti-SARS-CoV-2 Agents.ACS Med Chem Lett. 2024 Mar 1;15(5):602-609. doi: 10.1021/acsmedchemlett.3c00562. eCollection 2024 May 9. ACS Med Chem Lett. 2024. PMID: 38746883
References
-
- Backer V., Sjobring U., Sonne J., Weiss A., Hostrup M., Johansen H.K., Becker V., Sonne D.P., Balchen T., Jellingso M., Sommer M.O.A. A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: a broad spectrum antiviral candidate for treatment of COVID-19. The Lancet regional health. Europe. 2021;4:100084. - PMC - PubMed
-
- Banerji V., Frumm S.M., Ross K.N., Li L.S., Schinzel A.C., Hahn C.K., Kakoza R.M., Chow K.T., Ross L., Alexe G., Tolliday N., Inguilizian H., Galinsky I., Stone R.M., DeAngelo D.J., Roti G., Aster J.C., Hahn W.C., Kung A.L., Stegmaier K. The intersection of genetic and chemical genomic screens identifies GSK-3α as a target in human acute myeloid leukemia. J. Clin. Invest. 2012;122:935–947. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous