DHA-enriched phosphatidylcholine suppressed angiogenesis by activating PPARγ and modulating the VEGFR2/Ras/ERK pathway in human umbilical vein endothelial cells
- PMID: 34868703
- PMCID: PMC8595343
- DOI: 10.1007/s10068-021-00990-0
DHA-enriched phosphatidylcholine suppressed angiogenesis by activating PPARγ and modulating the VEGFR2/Ras/ERK pathway in human umbilical vein endothelial cells
Abstract
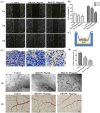
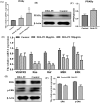
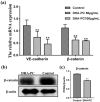
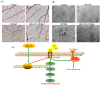
Docosahexaenoic acid-enriched phosphatidylcholine (DHA-PC) is a new generation of omega-3 lipids, which contains an ester bond linking DHA at the sn-2 position of phospholipid. DHA-PC has become the interest recently as its better bioavailability and anti-oxidation capacity. In this study, the anti-angiogenic effect of DHA-PC was evaluated. The capacities of proliferation, migration, tube formation of human umbilical vein endothelial cells were significantly declined after DHA-PC treatment. Furthermore, DHA-PC inhibited the neovascularization of the chick chorioallantoic membrane in vivo. Mechanism results indicated that DHA-PC enhances the expression of peroxisome proliferator-activated receptor γ (PPARγ) at transcriptional and translational level, subsequently down-regulates the VEGFR2 expression and VEGFR2-mediated downstream Ras/ERK pathway, resulting in significant reduction in proliferation and differentiation. Additionally, PPARγ-specific antagonist GW9662 partly reversed the inhibition effects of DHA-PC on tube formation and neovascularization, suggesting that DHA-PC exerts anti-angiogenesis effect through activating PPARγ. These findings indicated that DHA-PC has a great prospect of anti-tumor angiogenesis therapy.
Keywords: Angiogenesis; DHA-PC; Neovascularization; PPARγ; Tube formation.
© The Korean Society of Food Science and Technology 2021.
Conflict of interest statement
Conflict of interestNo potential conflicts of interest.
Figures

Similar articles
-
DHA- and EPA-Enriched Phosphatidylcholine Suppress Human Lung Carcinoma 95D Cells Metastasis via Activating the Peroxisome Proliferator-Activated Receptor γ.Nutrients. 2022 Nov 4;14(21):4675. doi: 10.3390/nu14214675. Nutrients. 2022. PMID: 36364935 Free PMC article.
-
DHA/EPA-Enriched Phosphatidylcholine Suppresses Tumor Growth and Metastasis via Activating Peroxisome Proliferator-Activated Receptor γ in Lewis Lung Cancer Mice.J Agric Food Chem. 2021 Jan 20;69(2):676-685. doi: 10.1021/acs.jafc.0c06890. Epub 2021 Jan 6. J Agric Food Chem. 2021. PMID: 33406839
-
Dihydroartemisinin targets VEGFR2 via the NF-κB pathway in endothelial cells to inhibit angiogenesis.Cancer Biol Ther. 2014;15(11):1479-88. doi: 10.4161/15384047.2014.955728. Cancer Biol Ther. 2014. PMID: 25482945 Free PMC article.
-
Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells.Res Vet Sci. 2017 Jun;112:7-12. doi: 10.1016/j.rvsc.2016.12.011. Epub 2017 Jan 5. Res Vet Sci. 2017. PMID: 28095338 Review.
-
The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina.Prog Retin Eye Res. 2005 Jan;24(1):87-138. doi: 10.1016/j.preteyeres.2004.06.002. Prog Retin Eye Res. 2005. PMID: 15555528 Review.
Cited by
-
Comparative Study of Docosahexaenoic Acid with Different Molecular Forms for Promoting Apoptosis of the 95D Non-Small-Cell Lung Cancer Cells in a PPARγ-Dependent Manner.Mar Drugs. 2022 Sep 23;20(10):599. doi: 10.3390/md20100599. Mar Drugs. 2022. PMID: 36286423 Free PMC article.
-
Polysaccharide dextran-based conjugate for selective co-delivery of two synergistic drugs docetaxel and docosahexaenoic acid to tumor cells.Drug Deliv. 2023 Dec;30(1):40-50. doi: 10.1080/10717544.2022.2152133. Drug Deliv. 2023. PMID: 36458324 Free PMC article.
-
Molecular Regulation of Differential Lipid Molecule Accumulation in the Intramuscular Fat and Abdominal Fat of Chickens.Genes (Basel). 2023 Jul 17;14(7):1457. doi: 10.3390/genes14071457. Genes (Basel). 2023. PMID: 37510361 Free PMC article.
-
The Landscape of Lipid Metabolism in Lung Cancer: The Role of Structural Profiling.J Clin Med. 2023 Feb 21;12(5):1736. doi: 10.3390/jcm12051736. J Clin Med. 2023. PMID: 36902523 Free PMC article.
References
-
- Aljada A, O'Connor L, Fu YY, Mousa SA. PPAR gamma ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesis. Angiogenesis. 2008;11:361–367. - PubMed
-
- Berger J, Moller DE. The mechanisms of action of PPARs. Annual Review of Medicine. 2002;53:409–435. - PubMed
-
- Corton JC, Anderson SP, Stauber A. Central role of peroxisome proliferator-activated receptors in the actions of peroxisome proliferators. Annual Review of Pharmacology and Toxicology. 2000;40:491–518. - PubMed
-
- Ding S, Merkulova-Rainon T, Han ZC, Tobelem G. HGF receptor up-regulation contributes to the angiogenic phenotype of human endothelial cells and promotes angiogenesis in vitro. Blood. 2003;101:4816–4822. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
