20-HETE Participates in Intracerebral Hemorrhage-Induced Acute Injury by Promoting Cell Ferroptosis
- PMID: 34867747
- PMCID: PMC8633108
- DOI: 10.3389/fneur.2021.763419
20-HETE Participates in Intracerebral Hemorrhage-Induced Acute Injury by Promoting Cell Ferroptosis
Abstract
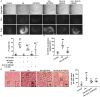
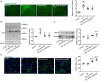
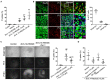
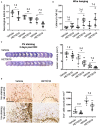
Intracerebral hemorrhage (ICH) is a highly fatal type of stroke that leads to various types of neuronal death. Recently, ferroptosis, a form of cell death resulting from iron-dependent lipid peroxide accumulation, was observed in a mouse ICH model. N-hydroxy-N'-(4-n-butyl-2-methylphenyl)-formamidine (HET0016), which inhibits synthesis of the arachidonic acid metabolite 20-hydroxyeicosatetraenoic acid (20-HETE), has shown a protective effect after ICH. However, the underlying mechanisms of the neuroprotective effect need further investigation. We explored whether 20-HETE participates in ICH-induced ferroptosis ex vivo by using hemoglobin-treated organotypic hippocampal slice cultures (OHSCs) and in vivo by using a collagenase-induced ICH mouse model. Ex vivo, we found that the 20-HETE synthesis inhibitor HET0016 and antagonist 20-6,15-HEDGE reduced hemoglobin-induced cell death, iron deposition, and lipid reactive oxygen species levels in OHSCs. Furthermore, 20-HETE inhibition in OHSCs increased the expression of glutathione peroxidase (GPX) 4, an antioxidant enzyme that serves as a main regulator of ferroptosis. In contrast, exposure of OHSCs to the 20-HETE stable mimetic 20-5,14-HEDGE induced cell death that was significantly inhibited by the ferroptosis inhibitor ferrostatin-1. In vivo, HET0016 treatment ameliorated focal deficits, reduced lesion volume, and decreased iron accumulation around the lesion at day 3 and 7 after ICH. In addition, lipid peroxidation was decreased and expression of GPX4 was increased in the HET0016-treated ICH group. The mitogen-activated protein kinase pathway also was inhibited by HET0016 in vivo. These results indicate that 20-HETE contributes to ICH-induced acute brain injury in part by activating ferroptosis pathways, thereby providing an upstream target for inhibiting ferroptosis.
Keywords: 20-hydroxyeicosatetraenoic acid; ferroptosis; glutathione peroxidase; intracerebral hemorrhage; lipid peroxide.
Copyright © 2021 Han, Wan, Han, Ren, Falck, Munnuri, Yang and Koehler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
20-HETE synthesis inhibition promotes cerebral protection after intracerebral hemorrhage without inhibiting angiogenesis.J Cereb Blood Flow Metab. 2019 Aug;39(8):1531-1543. doi: 10.1177/0271678X18762645. Epub 2018 Feb 27. J Cereb Blood Flow Metab. 2019. PMID: 29485354 Free PMC article.
-
Inhibition of neuronal ferroptosis protects hemorrhagic brain.JCI Insight. 2017 Apr 6;2(7):e90777. doi: 10.1172/jci.insight.90777. JCI Insight. 2017. PMID: 28405617 Free PMC article.
-
Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage.Brain Res. 2018 Dec 15;1701:112-125. doi: 10.1016/j.brainres.2018.09.012. Epub 2018 Sep 8. Brain Res. 2018. PMID: 30205109
-
Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage.Stroke Vasc Neurol. 2019 Jan 13;4(2):93-95. doi: 10.1136/svn-2018-000205. eCollection 2019 Jul. Stroke Vasc Neurol. 2019. PMID: 31338218 Free PMC article. Review.
-
Ferroptosis, a Regulated Neuronal Cell Death Type After Intracerebral Hemorrhage.Front Cell Neurosci. 2020 Nov 16;14:591874. doi: 10.3389/fncel.2020.591874. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33304242 Free PMC article. Review.
Cited by
-
The interaction between STING and NCOA4 exacerbates lethal sepsis by orchestrating ferroptosis and inflammatory responses in macrophages.Cell Death Dis. 2022 Jul 28;13(7):653. doi: 10.1038/s41419-022-05115-x. Cell Death Dis. 2022. PMID: 35902564 Free PMC article.
-
Dexpramipexole Attenuates White Matter Injury to Facilitate Locomotion and Motor Coordination Recovery via Reducing Ferroptosis after Intracerebral Hemorrhage.Oxid Med Cell Longev. 2022 Aug 4;2022:6160701. doi: 10.1155/2022/6160701. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35965685 Free PMC article.
-
Elucidating the progress and impact of ferroptosis in hemorrhagic stroke.Front Cell Neurosci. 2023 Jan 11;16:1067570. doi: 10.3389/fncel.2022.1067570. eCollection 2022. Front Cell Neurosci. 2023. PMID: 36713782 Free PMC article. Review.
-
The Regulated Cell Death and Potential Interventions in Preterm Infants after Intracerebral Hemorrhage.Curr Neuropharmacol. 2023;21(7):1488-1503. doi: 10.2174/1570159X21666221117155209. Curr Neuropharmacol. 2023. PMID: 36397619 Free PMC article. Review.
-
Targeting Ferroptosis Promotes Functional Recovery by Mitigating White Matter Injury Following Acute Carbon Monoxide Poisoning.Mol Neurobiol. 2024 Feb;61(2):1157-1174. doi: 10.1007/s12035-023-03603-5. Epub 2023 Sep 11. Mol Neurobiol. 2024. PMID: 37697220
References
Grants and funding
LinkOut - more resources
Full Text Sources

