Antinociceptive and Antipruritic Effects of HSK21542, a Peripherally-Restricted Kappa Opioid Receptor Agonist, in Animal Models of Pain and Itch
- PMID: 34867403
- PMCID: PMC8635029
- DOI: 10.3389/fphar.2021.773204
Antinociceptive and Antipruritic Effects of HSK21542, a Peripherally-Restricted Kappa Opioid Receptor Agonist, in Animal Models of Pain and Itch
Abstract
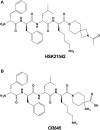
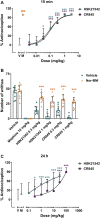
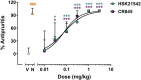
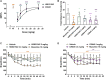
Kappa opioid receptor (KOR) agonists have been promising therapeutic candidates, owing to their potential for relieving pain and treating intractable pruritus. Although lacking morphine-like central nervous system (CNS) effects, KOR agonists do elicit sedation, dysphoria and diuresis which seriously impede their development. Peripherally-restricted KOR agonists have a poor ability to penetrate into the CNS system, so that CNS-related adverse effects can be ameliorated or even abolished. However, the only approved peripherally-restricted KOR agonist CR845 remains some frequent CNS adverse events. In the present study, we aim to address pharmacological profiles of HSK21542, with an expectation to provide a safe and effective alternative for patients who are suffering from pain and pruritus. The in vitro experimental results showed that HSK21542 was a selective and potent KOR agonist with higher potency than CR845, and had a brain/plasma concentration ratio of 0.001, indicating its peripheral selectivity. In animal models of pain, HSK21542 significantly inhibited acetic acid-, hindpaw incision- or chronic constriction injury-induced pain-related behaviors, and the efficacy was comparable to CR845 at 15 min post-dosing. HSK21542 had a long-lasting analgesic potency with a median effective dose of 1.48 mg/kg at 24 h post-drug in writhing test. Meanwhile, the antinociceptive activity of HSK21542 was effectively reversed by a KOR antagonist nor-binaltorphimine. In addition, HSK21542 had powerful antipruritic activities in compound 48/80-induced itch model. On the other hand, HSK21542 had a weak ability to produce central antinociceptive effects in a hot-plate test and fewer effects on the locomotor activity of mice. HSK21542 didn't affect the respiratory rate of mice. Therefore, HSK21542 might be a safe and effective KOR agonist and promising candidate for treating pain and pruritus.
Keywords: HSK21542; animal models; kappa opioid receptor; pain; pruritus.
Copyright © 2021 Wang, Gou, Yu, Bai, Tan, Cao, Qian, Zheng, Wang, Tang, Zhang, Ye and Ni.
Conflict of interest statement
Authors XG, XY, DB, BT, PC, MQ, XZ, HW, PT, CZ, FY, and JN are employed by Haisco Pharmaceutical Group Co., Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The Kappa Opioid Receptor: A Promising Therapeutic Target for Multiple Pathologies.Front Pharmacol. 2022 Jun 20;13:837671. doi: 10.3389/fphar.2022.837671. eCollection 2022. Front Pharmacol. 2022. PMID: 35795569 Free PMC article. Review.
-
ZYKR1, a novel, potent, and peripherally selective kappa opioid receptor agonist reduces visceral pain and pruritus in animal models.Eur J Pharmacol. 2022 Jun 5;924:174961. doi: 10.1016/j.ejphar.2022.174961. Epub 2022 Apr 17. Eur J Pharmacol. 2022. PMID: 35443192
-
Conorphin-66 produces peripherally restricted antinociception via the kappa-opioid receptor with limited side effects.Neuropharmacology. 2024 Dec 15;261:110157. doi: 10.1016/j.neuropharm.2024.110157. Epub 2024 Sep 12. Neuropharmacology. 2024. PMID: 39276862
-
Effects of Intrathecal κ-Opioid Receptor Agonist on Morphine-Induced Itch and Antinociception in Mice.Reg Anesth Pain Med. 2016 Jan-Feb;41(1):69-74. doi: 10.1097/AAP.0000000000000326. Reg Anesth Pain Med. 2016. PMID: 26587674
-
Signaling underlying kappa opioid receptor-mediated behaviors in rodents.Front Neurosci. 2022 Nov 3;16:964724. doi: 10.3389/fnins.2022.964724. eCollection 2022. Front Neurosci. 2022. PMID: 36408401 Free PMC article. Review.
Cited by
-
The Kappa Opioid Receptor: A Promising Therapeutic Target for Multiple Pathologies.Front Pharmacol. 2022 Jun 20;13:837671. doi: 10.3389/fphar.2022.837671. eCollection 2022. Front Pharmacol. 2022. PMID: 35795569 Free PMC article. Review.
-
Targeting sensory neuron GPCRs for peripheral neuropathic pain.Trends Pharmacol Sci. 2023 Dec;44(12):1009-1027. doi: 10.1016/j.tips.2023.10.003. Epub 2023 Nov 11. Trends Pharmacol Sci. 2023. PMID: 37977131 Free PMC article. Review.
-
Druggable Targets and Compounds with Both Antinociceptive and Antipruritic Effects.Pharmaceuticals (Basel). 2022 Jul 19;15(7):892. doi: 10.3390/ph15070892. Pharmaceuticals (Basel). 2022. PMID: 35890193 Free PMC article. Review.
-
Discovery of κ Opioid Receptor (KOR)-Selective d-Tetrapeptides with Improved In Vivo Antinociceptive Effect after Peripheral Administration.ACS Med Chem Lett. 2022 Oct 17;13(11):1707-1714. doi: 10.1021/acsmedchemlett.2c00237. eCollection 2022 Nov 10. ACS Med Chem Lett. 2022. PMID: 36385929 Free PMC article.
-
Role of kappa-opioid and mu-opioid receptors in pruritus: Peripheral and central itch circuits.Exp Dermatol. 2022 Dec;31(12):1900-1907. doi: 10.1111/exd.14669. Epub 2022 Sep 23. Exp Dermatol. 2022. PMID: 36054458 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

