The Role of Mitochondria in Optic Atrophy With Autosomal Inheritance
- PMID: 34867178
- PMCID: PMC8634724
- DOI: 10.3389/fnins.2021.784987
The Role of Mitochondria in Optic Atrophy With Autosomal Inheritance
Abstract
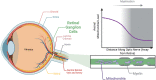
Optic atrophy (OA) with autosomal inheritance is a form of optic neuropathy characterized by the progressive and irreversible loss of vision. In some cases, this is accompanied by additional, typically neurological, extra-ocular symptoms. Underlying the loss of vision is the specific degeneration of the retinal ganglion cells (RGCs) which form the optic nerve. Whilst autosomal OA is genetically heterogenous, all currently identified causative genes appear to be associated with mitochondrial organization and function. However, it is unclear why RGCs are particularly vulnerable to mitochondrial aberration. Despite the relatively high prevalence of this disorder, there are currently no approved treatments. Combined with the lack of knowledge concerning the mechanisms through which aberrant mitochondrial function leads to RGC death, there remains a clear need for further research to identify the underlying mechanisms and develop treatments for this condition. This review summarizes the genes known to be causative of autosomal OA and the mitochondrial dysfunction caused by pathogenic mutations. Furthermore, we discuss the suitability of available in vivo models for autosomal OA with regards to both treatment development and furthering the understanding of autosomal OA pathology.
Keywords: in vivo models; mitochondria; optic atrophy; retinal ganglion cells (RGC); retinal organoids.
Copyright © 2021 Strachan, Mac White-Begg, Crean, Reynolds, Kennedy and O’Sullivan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Role of Oxidative Stress in Ocular Diseases Associated with Retinal Ganglion Cells Degeneration.Antioxidants (Basel). 2021 Dec 5;10(12):1948. doi: 10.3390/antiox10121948. Antioxidants (Basel). 2021. PMID: 34943051 Free PMC article. Review.
-
Dominant optic atrophy.Orphanet J Rare Dis. 2012 Jul 9;7:46. doi: 10.1186/1750-1172-7-46. Orphanet J Rare Dis. 2012. PMID: 22776096 Free PMC article. Review.
-
Dominant optic atrophy: Culprit mitochondria in the optic nerve.Prog Retin Eye Res. 2021 Jul;83:100935. doi: 10.1016/j.preteyeres.2020.100935. Epub 2020 Dec 17. Prog Retin Eye Res. 2021. PMID: 33340656 Review.
-
New avenues for therapy in mitochondrial optic neuropathies.Ther Adv Rare Dis. 2021 Jul 19;2:26330040211029037. doi: 10.1177/26330040211029037. eCollection 2021 Jan-Dec. Ther Adv Rare Dis. 2021. PMID: 37181108 Free PMC article. Review.
-
Wolfram syndrome: new pathophysiological insights and therapeutic strategies.Ther Adv Rare Dis. 2021 Aug 16;2:26330040211039518. doi: 10.1177/26330040211039518. eCollection 2021 Jan-Dec. Ther Adv Rare Dis. 2021. PMID: 37181110 Free PMC article. Review.
Cited by
-
Mitochondria in Retinal Ganglion Cells: Unraveling the Metabolic Nexus and Oxidative Stress.Int J Mol Sci. 2024 Aug 7;25(16):8626. doi: 10.3390/ijms25168626. Int J Mol Sci. 2024. PMID: 39201313 Free PMC article. Review.
-
Mitophagy in Astrocytes Is Required for the Health of Optic Nerve.Cells. 2023 Oct 20;12(20):2496. doi: 10.3390/cells12202496. Cells. 2023. PMID: 37887340 Free PMC article.
-
Pyrroloquinoline quinone drives ATP synthesis in vitro and in vivo and provides retinal ganglion cell neuroprotection.Acta Neuropathol Commun. 2023 Sep 8;11(1):146. doi: 10.1186/s40478-023-01642-6. Acta Neuropathol Commun. 2023. PMID: 37684640 Free PMC article.
-
Optimisation of AAV-NDI1 Significantly Enhances Its Therapeutic Value for Correcting Retinal Mitochondrial Dysfunction.Pharmaceutics. 2023 Jan 18;15(2):322. doi: 10.3390/pharmaceutics15020322. Pharmaceutics. 2023. PMID: 36839646 Free PMC article.
References
-
- Ali M. S., Suda K., Kowada R., Ueoka I., Yoshida H., Yamaguchi M. (2020). Neuron-specific knockdown of solute carrier protein SLC25A46a induces locomotive defects, an abnormal neuron terminal morphology, learning disability, and shortened lifespan. IBRO Rep. 8 65–75. 10.1016/j.ibror.2020.02.001 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

