Loss of Hepatic Surf4 Depletes Lipid Droplets in the Adrenal Cortex but Does Not Impair Adrenal Hormone Production
- PMID: 34859075
- PMCID: PMC8631933
- DOI: 10.3389/fcvm.2021.764024
Loss of Hepatic Surf4 Depletes Lipid Droplets in the Adrenal Cortex but Does Not Impair Adrenal Hormone Production
Erratum in
-
Corrigendum: Loss of hepatic Surf4 depletes lipid droplets in the adrenal cortex but does not impair adrenal hormone production.Front Cardiovasc Med. 2023 May 10;10:1215335. doi: 10.3389/fcvm.2023.1215335. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37234368 Free PMC article.
Abstract
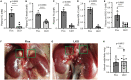
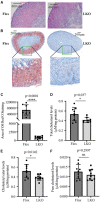
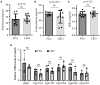
The adrenal gland produces steroid hormones to play essential roles in regulating various physiological processes. Our previous studies showed that knockout of hepatic Surf4 (Surf4LKO) markedly reduced fasting plasma total cholesterol levels in adult mice, including low-density lipoprotein and high-density lipoprotein cholesterol. Here, we found that plasma cholesterol levels were also dramatically reduced in 4-week-old young mice and non-fasted adult mice. Circulating lipoprotein cholesterol is an important source of the substrate for the production of adrenal steroid hormones. Therefore, we investigated whether adrenal steroid hormone production was affected in Surf4LKO mice. We observed that lacking hepatic Surf4 essentially eliminated lipid droplets and significantly reduced cholesterol levels in the adrenal gland; however, plasma levels of aldosterone and corticosterone were comparable in Surf4LKO and the control mice under basal and stress conditions. Further analysis revealed that mRNA levels of genes encoding enzymes important for hormone synthesis were not altered, whereas the expression of scavenger receptor class B type I (SR-BI), low-density lipoprotein receptor (LDLR) and 3-hydroxy-3-methyl-glutaryl-CoA reductase was significantly increased in the adrenal gland of Surf4LKO mice, indicating increased de novo cholesterol biosynthesis and enhanced LDLR and SR-BI-mediated lipoprotein cholesterol uptake. We also observed that the nuclear form of SREBP2 was increased in the adrenal gland of Surf4 LKO mice. Taken together, these findings indicate that the very low levels of circulating lipoprotein cholesterol in Surf4LKO mice cause a significant reduction in adrenal cholesterol levels but do not significantly affect adrenal steroid hormone production. Reduced adrenal cholesterol levels activate SREBP2 and thus increase the expression of genes involved in cholesterol biosynthesis, which increases de novo cholesterol synthesis to compensate for the loss of circulating lipoprotein-derived cholesterol in the adrenal gland of Surf4LKO mice.
Keywords: LDL receptor (LDLR); LDL–cholesterol; atherosclerosis; cholesterol; proprotein convertase subtilisin/kexin 9; triglyceride.
Copyright © 2021 Chang, Zhao, Qin, Wang, Wang, Zhai, Liu, Gu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
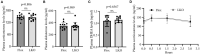
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

