Challenges and Opportunities for Preexposure Prophylaxis
- PMID: 34856093
- PMCID: PMC8670824
Challenges and Opportunities for Preexposure Prophylaxis
Abstract
Despite major advances in the HIV prevention toolbox in the past decade, there remain substantial social, economic, and structural barriers to access to preexposure prophylaxis (PrEP) that prevent a universal, population-level reduction in HIV incidence. Daily oral tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) has been the flagship PrEP regimen, and data support a pericoital/on-demand "2-1-1" dosing schedule for men who have sex with men. Daily oral PrEP with tenofovir alafenamide combined with emtricitabine (TAF/FTC) was approved by the US Food and Drug Administration (FDA) in 2019 for all routes of exposure other than vaginal exposures. The effectiveness of daily oral TDF/FTC has not been consistent in cisgender women outside of serodifferent couples, likely owing to differences in vaginal tissue penetration of PrEP agents resulting in less "forgiveness" of nonadherence. These observations have highlighted the need for additional choices of HIV prevention strategies. Injectable long-acting cabotegravir was recently shown to be superior to daily oral TDF/FTC across risk populations. PrEP studies of islatravir are underway for a monthly oral formulation and a drug-eluting subdermal implant. Lenacapavir, with a novel mechanism of action, is under investigation as a subcutaneous injection at 6-month intervals.
Figures

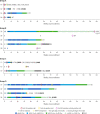
Similar articles
-
Preexposure Prophylaxis for the Prevention of HIV: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force.JAMA. 2023 Aug 22;330(8):746-763. doi: 10.1001/jama.2023.9865. JAMA. 2023. PMID: 37606667
-
Cost-Effectiveness of Long-Acting Injectable HIV Preexposure Prophylaxis in the United States : A Cost-Effectiveness Analysis.Ann Intern Med. 2022 Apr;175(4):479-489. doi: 10.7326/M21-1548. Epub 2022 Feb 1. Ann Intern Med. 2022. PMID: 35099992 Free PMC article.
-
Cabotegravir Extended-Release Injectable Suspension: A Review in HIV-1 Pre-Exposure Prophylaxis.Drugs. 2022 Sep;82(14):1489-1498. doi: 10.1007/s40265-022-01791-3. Epub 2022 Oct 18. Drugs. 2022. PMID: 36255686 Review.
-
PrEP Demonstration Project Showed Superior Adherence with Tenofovir Alafenamide/Emtricitabine Compared to Tenofovir Disoproxil Fumarate/Emtricitabine in a Sample of Partnered Sexual Minority Men.AIDS Behav. 2021 Apr;25(4):1299-1305. doi: 10.1007/s10461-020-03095-7. Epub 2020 Nov 18. AIDS Behav. 2021. PMID: 33206262 Free PMC article.
-
Personalizing prevention: Advances in pharmacotherapy for HIV prevention.Pharmacotherapy. 2023 Apr;43(4):305-320. doi: 10.1002/phar.2796. Epub 2023 Apr 1. Pharmacotherapy. 2023. PMID: 36938645 Review.
Cited by
-
Physiologically Based Pharmacokinetic Modelling of Cabotegravir Microarray Patches in Rats and Humans.Pharmaceutics. 2023 Nov 30;15(12):2709. doi: 10.3390/pharmaceutics15122709. Pharmaceutics. 2023. PMID: 38140050 Free PMC article.
-
Awareness of Heightened Sexual and Behavioral Vulnerability as a Trigger for PrEP Resumption Among Adolescent Girls and Young Women in East and Southern Africa.Curr HIV/AIDS Rep. 2023 Dec;20(6):333-344. doi: 10.1007/s11904-023-00680-y. Epub 2023 Dec 5. Curr HIV/AIDS Rep. 2023. PMID: 38051383 Free PMC article. Review.
-
From Innovation to Implementation: The Evolution of HIV Pre-Exposure Prophylaxis and Future Implications.Pathogens. 2023 Jul 9;12(7):924. doi: 10.3390/pathogens12070924. Pathogens. 2023. PMID: 37513771 Free PMC article. Review.
-
Clinical Considerations in the Selection of Preexposure Prophylaxis for HIV Prevention in Canada.Can J Infect Dis Med Microbiol. 2022 Aug 30;2022:3913439. doi: 10.1155/2022/3913439. eCollection 2022. Can J Infect Dis Med Microbiol. 2022. PMID: 36081603 Free PMC article. Review.
-
Long-Acting Injectable Human Immunodeficiency Virus Pre-Exposure Prophylaxis Preferred Over Other Modalities Among People Who Inject Drugs: Findings from a Qualitative Study in California.AIDS Patient Care STDS. 2022 Jul;36(7):254-262. doi: 10.1089/apc.2022.0068. Epub 2022 Jun 21. AIDS Patient Care STDS. 2022. PMID: 35727647 Free PMC article.
References
-
- Coelho LE, Torres TS, Veloso VG, Landovitz RJ, Grinsztejn B. Pre-exposure prophylaxis 2.0: new drugs and technologies in the pipeline. Lancet HIV. 2019;6(11):e788–e799. - PubMed
-
- UNAIDS. UNAIDS global AIDS update 2020. https://www.unaids.org/sites/default/files/media_asset/2020_global-aids-.... Accessed on August 15, 2021.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
